Search by Drug Name or NDC
NDC 51672-4213-07 Iloperidone Details
Iloperidone
Iloperidone is a KIT in the HUMAN PRESCRIPTION DRUG category. It is labeled and distributed by Taro Pharmaceuticals U.S.A., Inc.. The primary component is .
MedlinePlus Drug Summary
Iloperidone is used to treat the symptoms of schizophrenia (a mental illness that causes disturbed or unusual thinking, loss of interest in life, and strong or inappropriate emotions). Iloperidone is in a class of medications called atypical antipsychotics. It works by changing the activity of certain natural substances in the brain.
Related Packages: 51672-4213-07Last Updated: 11/30/2022
MedLinePlus Full Drug Details: Iloperidone
Product Information
| NDC | 51672-4213 |
|---|---|
| Product ID | 51672-4213_6787555e-8a11-481c-b05c-179b0aedcf5c |
| Associated GPIs | |
| GCN Sequence Number | n/a |
| GCN Sequence Number Description | n/a |
| HIC3 | n/a |
| HIC3 Description | n/a |
| GCN | n/a |
| HICL Sequence Number | n/a |
| HICL Sequence Number Description | n/a |
| Brand/Generic | n/a |
| Proprietary Name | Iloperidone |
| Proprietary Name Suffix | n/a |
| Non-Proprietary Name | Iloperidone |
| Product Type | HUMAN PRESCRIPTION DRUG |
| Dosage Form | KIT |
| Route | n/a |
| Active Ingredient Strength | n/a |
| Active Ingredient Units | n/a |
| Substance Name | n/a |
| Labeler Name | Taro Pharmaceuticals U.S.A., Inc. |
| Pharmaceutical Class | n/a |
| DEA Schedule | n/a |
| Marketing Category | ANDA |
| Application Number | ANDA207098 |
| Listing Certified Through | 2024-12-31 |
Package
Package Images








NDC 51672-4213-07 (51672421307)
| NDC Package Code | 51672-4213-7 |
|---|---|
| Billing NDC | 51672421307 |
| Package | 1 BLISTER PACK in 1 DOSE PACK (51672-4213-7) / 1 KIT in 1 BLISTER PACK |
| Marketing Start Date | 2019-07-22 |
| NDC Exclude Flag | N |
| Pricing Information | N/A |
Standard Product Labeling (SPL)/Prescribing Information SPL 6f17cc91-86b3-42e3-9bf2-935dd360c3eb Details
HIGHLIGHTS OF PRESCRIBING INFORMATION
ILOPERIDONE tablets, for oral use
Initial U.S. Approval: 2009
WARNING: INCREASED MORTALITY IN ELDERLY PATIENTS WITH DEMENTIA-RELATED PSYCHOSIS
See full prescribing information for complete boxed warning.
Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death. Iloperidone tablets are not approved for use in patients with dementia-related psychosis. (5.1)
RECENT MAJOR CHANGES
INDICATIONS AND USAGE
Iloperidone is an atypical antipsychotic indicated for the treatment of schizophrenia in adults. (1, 14) In choosing among treatments, prescribers should consider the ability of iloperidone to prolong the QT interval and the use of other drugs first. Prescribers should also consider the need to titrate iloperidone slowly to avoid orthostatic hypotension, which may lead to delayed effectiveness compared to some other drugs that do not require similar titration. (2, 5, 14)
DOSAGE AND ADMINISTRATION
The recommended target dosage of iloperidone tablets is 12 mg/day to 24 mg/day administered twice daily. This target dosage range is achieved by daily dosage adjustments, alerting patients to symptoms of orthostatic hypotension, starting at a dose of 1 mg twice daily, then moving to 2 mg, 4 mg, 6 mg, 8 mg, 10 mg, and 12 mg twice daily on Days 2, 3, 4, 5, 6, and 7 respectively, to reach the 12 mg/day to 24 mg/day dose range. Iloperidone tablets can be administered without regard to meals. (2.1)
DOSAGE FORMS AND STRENGTHS
1 mg, 2 mg, 4 mg, 6 mg, 8 mg, 10 mg and 12 mg tablets. (3)
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- Cerebrovascular Adverse Reactions in Elderly Patients with Dementia-Related Psychosis: Increased incidence of cerebrovascular adverse reactions (e.g., stroke, transient ischemic attack). (5.2)
- QT prolongation: Prolongs QT interval and may be associated with arrhythmia and sudden death—consider using other antipsychotics first. Avoid use of iloperidone tablets in combination with other drugs that are known to prolong QTc; use caution and consider dose modification when prescribing iloperidone tablets with other drugs that inhibit iloperidone metabolism. Monitor serum potassium and magnesium in patients at risk for electrolyte disturbances. (1, 5.3, 7.1, 7.3, 12.3)
- Neuroleptic Malignant Syndrome: Manage with immediate discontinuation of drug and close monitoring. (5.4)
- Tardive dyskinesia: Discontinue if clinically appropriate. (5.5)
- Metabolic Changes: Monitor for hyperglycemia/diabetes mellitus, dyslipidemia and weight gain. (5.6)
- Seizures: Use cautiously in patients with a history of seizures or with conditions that lower seizure threshold. (5.7)
- Orthostatic hypotension: Dizziness, tachycardia, and syncope can occur with standing. (5.8)
- Leukopenia, Neutropenia, and Agranulocytosis have been reported with antipsychotics. Patients with a pre-existing low white blood cell count (WBC) or a history of leukopenia/neutropenia should have their complete blood count (CBC) monitored frequently during the first few months of therapy and should discontinue iloperidone tablets at the first sign of a decline in WBC in the absence of other causative factors. (5.10)
- Suicide: Close supervision of high risk patients. (5.14)
- Priapism: Cases have been reported in association with iloperidone tablet treatment. (5.15)
- Potential for cognitive and motor impairment: Use caution when operating machinery. (5.16)
ADVERSE REACTIONS
Commonly observed adverse reactions (incidence ≥5% and 2-fold greater than placebo) were: dizziness, dry mouth, fatigue, nasal congestion, orthostatic hypotension, somnolence, tachycardia, and weight increased. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Taro Pharmaceuticals U.S.A., Inc. at 1-866-923-4914 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
The dose of iloperidone tablets should be reduced in patients coadministered a strong CYP2D6 or CYP3A4 inhibitor. (2.2, 7.1)
USE IN SPECIFIC POPULATIONS
- Pregnancy: May cause extrapyramidal and/or withdrawal symptoms in neonates with third trimester exposure. (8)
- Lactation: Advise not to breast feed. (8.2)
- Pediatric Use: Safety and effectiveness not established in children and adolescents. (8.3)
- Hepatic Impairment: Iloperidone tablets are not recommended for patients with severe hepatic impairment. (2.2, 8.6)
- The dose of iloperidone tablets should be reduced in patients who are poor metabolizers of CYP2D6. (2.2, 12.3)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 3/2018
Table Of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: INCREASED MORTALITY IN ELDERLY PATIENTS WITH DEMENTIA-RELATED PSYCHOSIS
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Usual Dose
2.2 Dosage in Special Populations
2.3 Maintenance Treatment
2.4 Reinitiation of Treatment in Patients Previously Discontinued
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Increased Mortality in Elderly Patients with Dementia-Related Psychosis
5.2 Cerebrovascular Adverse Reactions, Including Stroke, in Elderly Patients with Dementia-Related Psychosis
5.3 QT Prolongation
5.4 Neuroleptic Malignant Syndrome (NMS)
5.5 Tardive Dyskinesia
5.6 Metabolic Changes
5.7 Seizures
5.8 Orthostatic Hypotension and Syncope
5.9 Falls
5.10 Leukopenia, Neutropenia and Agranulocytosis
5.11 Hyperprolactinemia
5.12 Body Temperature Regulation
5.13 Dysphagia
5.14 Suicide
5.15 Priapism
5.16 Potential for Cognitive and Motor Impairment
6 ADVERSE REACTIONS
6.1 Clinical Studies Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Potential for Other Drugs to Affect Iloperidone Tablets
7.2 Potential for Iloperidone Tablets to Affect Other Drugs
7.3 Drugs that Prolong the QT Interval
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Pediatric Use
8.4 Geriatric Use
8.5 Renal Impairment
8.6 Hepatic Impairment
8.7 Smoking Status
9 DRUG ABUSE AND DEPENDENCE
9.1 Controlled Substance
9.2 Abuse
10 OVERDOSAGE
10.1 Human Experience
10.2 Management of Overdose
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
WARNING
Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death. Iloperidone tablets are not approved for the treatment of patients with dementia-related psychosis [see Warnings and Precautions (5.1)].
1 INDICATIONS AND USAGE
Iloperidone tablets are indicated for the treatment of schizophrenia in adults.
When deciding among the alternative treatments available for this condition, the prescriber should consider the finding that iloperidone tablets are associated with prolongation of the QTc interval [see Warnings and Precautions (5.3)]. Prolongation of the QTc interval is associated in some other drugs with the ability to cause torsade de pointes-type arrhythmia, a potentially fatal polymorphic ventricular tachycardia which can result in sudden death. In many cases this would lead to the conclusion that other drugs should be tried first. Whether iloperidone tablets will cause torsade de pointes or increase the rate of sudden death is not yet known. Patients must be titrated to an effective dose of iloperidone tablets. Thus, control of symptoms may be delayed during the first 1 to 2 weeks of treatment compared to some other antipsychotic drugs that do not require a similar titration. Prescribers should be mindful of this delay when selecting an antipsychotic drug for the treatment of schizophrenia [see Dosage and Administration (2.1) and Clinical Studies (14)].
2 DOSAGE AND ADMINISTRATION
2.1 Usual Dose
Iloperidone tablets must be titrated slowly from a low starting dose to avoid orthostatic hypotension due to its alpha-adrenergic blocking properties. The recommended starting dose for iloperidone tablets is 1 mg orally twice daily. Dose increases to reach the target range of 6 mg to 12 mg twice daily (12 mg/day to 24 mg/day) may be made with daily dosage adjustments not to exceed 2 mg twice daily (4 mg/day). The maximum recommended dose is 12 mg twice daily (24 mg/day). Iloperidone doses above 24 mg/day have not been systematically evaluated in the clinical trials. Efficacy was demonstrated with iloperidone tablets in a dose range of 6 mg to 12 mg twice daily. Prescribers should be mindful of the fact that patients need to be titrated to an effective dose of iloperidone tablets. Thus, control of symptoms may be delayed during the first 1 week to 2 weeks of treatment compared to some other antipsychotic drugs that do not require similar titration. Prescribers should also be aware that some adverse effects associated with iloperidone tablet use are dose related [see Adverse Reactions (6.1)].
Iloperidone tablets can be administered without regard to meals.
2.2 Dosage in Special Populations
Dosage adjustment for patients taking iloperidone tablets concomitantly with potential CYP2D6 inhibitors: Iloperidone tablet dose should be reduced by one-half when administered concomitantly with strong CYP2D6 inhibitors such as fluoxetine or paroxetine. When the CYP2D6 inhibitor is withdrawn from the combination therapy, iloperidone tablet dose should then be increased to where it was before [see Drug Interactions (7)].
Dosage adjustment for patients taking iloperidone tablets concomitantly with potential CYP3A4 inhibitors: Iloperidone tablet dose should be reduced by one-half when administered concomitantly with strong CYP3A4 inhibitors such as ketoconazole or clarithromycin. When the CYP3A4 inhibitor is withdrawn from the combination therapy, iloperidone tablet dose should be increased to where it was before [see Drug Interactions (7)].
Dosage adjustment for patients taking iloperidone tablets who are poor metabolizers of CYP2D6: Iloperidone tablet dose should be reduced by one-half for poor metabolizers of CYP2D6 [see Clinical Pharmacology (12.3)].
Hepatic Impairment: No dose adjustment to iloperidone tablets is needed in patients with mild hepatic impairment. Patients with moderate hepatic impairment may require dose reduction, if clinically indicated. Iloperidone tablets are not recommended for patients with severe hepatic impairment [see Use in Specific Populations (8.6)].
2.3 Maintenance Treatment
In a longer-term study, iloperidone tablets were effective in delaying time to relapse in patients with schizophrenia who were stabilized on iloperidone tablets up to 24 mg/day [see Clinical Studies (14)]. Patients should be periodically reassessed to determine the need for maintenance treatment.
3 DOSAGE FORMS AND STRENGTHS
Iloperidone tablets are available in the following strengths: 1 mg, 2 mg, 4 mg, 6 mg, 8 mg, 10 mg, and 12 mg. The oval (1 mg) white tablets are engraved with "T" on one side and on the other side the tablet strength. The round (2 mg, 4 mg, 6 mg, 8 mg, 10 mg, or 12 mg) white tablets are engraved with "T" on one side and on the other side the tablet strength.
4 CONTRAINDICATIONS
Iloperidone tablets are contraindicated in individuals with a known hypersensitivity reaction to the product. Anaphylaxis, angioedema, and other hypersensitivity reactions have been reported [see Adverse Reactions (6.2)].
5 WARNINGS AND PRECAUTIONS
5.1 Increased Mortality in Elderly Patients with Dementia-Related Psychosis
Antipsychotic drugs increase the all-cause risk of death in elderly patients with dementia-related psychosis. Analyses of 17 dementia-related psychosis placebo-controlled trials (modal duration of 10 weeks and largely in patients taking atypical antipsychotic drugs) revealed a risk of death in the drug-treated patients of between 1.6 times to 1.7 times that in placebo-treated patients. Over the course of a typical 10-week controlled trial, the rate of death in drug-treated patients was about 4.5%, compared to a rate of about 2.6% in placebo-treated patients.
Although the causes of death were varied, most of the deaths appeared to be either cardiovascular (e.g., heart failure, sudden death) or infectious (e.g., pneumonia) in nature. Iloperidone tablets are not approved for the treatment of patients with dementia-related psychosis [see Boxed Warning, Warnings and Precautions (5.2)].
5.2 Cerebrovascular Adverse Reactions, Including Stroke, in Elderly Patients with Dementia-Related Psychosis
In placebo-controlled trials in elderly subjects with dementia, patients randomized to risperidone, aripiprazole, and olanzapine had a higher incidence of stroke and transient ischemic attack, including fatal stroke. Iloperidone tablets are not approved for the treatment of patients with dementia-related psychosis [see Boxed Warning, Warnings and Precautions (5.1)].
5.3 QT Prolongation
In an open-label QTc study in patients with schizophrenia or schizoaffective disorder (n=160), iloperidone was associated with QTc prolongation of 9 msec at an iloperidone dose of 12 mg twice daily. The effect of iloperidone on the QT interval was augmented by the presence of CYP450 2D6 or 3A4 metabolic inhibition (paroxetine 20 mg once daily and ketoconazole 200 mg twice daily, respectively). Under conditions of metabolic inhibition for both 2D6 and 3A4, iloperidone tablets 12 mg twice daily was associated with a mean QTcF increase from baseline of about 19 msec.
No cases of torsade de pointes or other severe cardiac arrhythmias were observed during the pre-marketing clinical program.
The use of iloperidone tablets should be avoided in combination with other drugs that are known to prolong QTc including Class 1A (e.g., quinidine, procainamide) or Class III (e.g., amiodarone, sotalol) antiarrhythmic medications, antipsychotic medications (e.g., chlorpromazine, thioridazine), antibiotics (e.g., gatifloxacin, moxifloxacin), or any other class of medications known to prolong the QTc interval (e.g., pentamidine, levomethadyl acetate, methadone). Iloperidone tablets should also be avoided in patients with a known genetic susceptibility to congenital long QT syndrome and in patients with a history of cardiac arrhythmias.
Certain circumstances may increase the risk of torsade de pointes and/or sudden death in association with the use of drugs that prolong the QTc interval, including (1) bradycardia; (2) hypokalemia or hypomagnesemia; (3) concomitant use of other drugs that prolong the QTc interval; and (4) presence of congenital prolongation of the QT interval; (5) recent acute myocardial infarction; and/or (6) uncompensated heart failure.
Caution is warranted when prescribing iloperidone tablets with drugs that inhibit iloperidone metabolism [see Drug Interactions (7.1)], and in patients with reduced activity of CYP2D6 [see Clinical Pharmacology (12.3)].
It is recommended that patients being considered for iloperidone tablet treatment who are at risk for significant electrolyte disturbances have baseline serum potassium and magnesium measurements with periodic monitoring. Hypokalemia (and/or hypomagnesemia) may increase the risk of QT prolongation and arrhythmia. Iloperidone tablets should be avoided in patients with histories of significant cardiovascular illness, e.g., QT prolongation, recent acute myocardial infarction, uncompensated heart failure, or cardiac arrhythmia. Iloperidone tablets should be discontinued in patients who are found to have persistent QTc measurements >500 msec.
If patients taking iloperidone tablets experience symptoms that could indicate the occurrence of cardiac arrhythmias, e.g., dizziness, palpitations, or syncope, the prescriber should initiate further evaluation, including cardiac monitoring.
5.4 Neuroleptic Malignant Syndrome (NMS)
A potentially fatal symptom complex sometimes referred to as Neuroleptic Malignant Syndrome (NMS) has been reported in association with administration of antipsychotic drugs, including iloperidone. Clinical manifestations include hyperpyrexia, muscle rigidity, altered mental status (including catatonic signs) and evidence of autonomic instability (irregular pulse or blood pressure, tachycardia, diaphoresis, and cardiac dysrhythmia). Additional signs may include elevated creatine phosphokinase, myoglobinuria (rhabdomyolysis), and acute renal failure.
The diagnostic evaluation of patients with this syndrome is complicated. In arriving at a diagnosis, it is important to identify cases in which the clinical presentation includes both serious medical illness (e.g., pneumonia, systemic infection, etc.) and untreated or inadequately treated extrapyramidal signs and symptoms (EPS). Other important considerations in the differential diagnosis include central anticholinergic toxicity, heat stroke, drug fever, and primary central nervous system (CNS) pathology.
The management of this syndrome should include: (1) immediate discontinuation of the antipsychotic drugs and other drugs not essential to concurrent therapy, (2) intensive symptomatic treatment and medical monitoring, and (3) treatment of any concomitant serious medical problems for which specific treatments are available. There is no general agreement about specific pharmacological treatment regimens for NMS.
If a patient requires antipsychotic drug treatment after recovery from NMS, the potential reintroduction of drug therapy should be carefully considered. The patient should be carefully monitored, since recurrences of NMS have been reported.
5.5 Tardive Dyskinesia
Tardive dyskinesia is a syndrome consisting of potentially irreversible, involuntary, dyskinetic movements, which may develop in patients treated with antipsychotic drugs. Although the prevalence of the syndrome appears to be highest among the elderly, especially elderly women, it is impossible to rely on prevalence estimates to predict, at the inception of antipsychotic treatment, which patients are likely to develop the syndrome. Whether antipsychotic drug products differ in their potential to cause tardive dyskinesia is unknown.
The risk of developing tardive dyskinesia and the likelihood that it will become irreversible are believed to increase as the duration of treatment and the total cumulative dose of antipsychotic administered increases. However, the syndrome can develop, although much less commonly, after relatively brief treatment periods at low doses.
There is no known treatment for established cases of tardive dyskinesia, although the syndrome may remit, partially or completely, if antipsychotic treatment is withdrawn. Antipsychotic treatment itself, however, may suppress (or partially suppress) the signs and symptoms of the syndrome and thereby may possibly mask the underlying process. The effect that symptomatic suppression has upon the long-term course of the syndrome is unknown. Given these considerations, iloperidone tablets should be prescribed in a manner that is most likely to minimize the occurrence of tardive dyskinesia. Chronic antipsychotic treatment should generally be reserved for patients who suffer from a chronic illness that (1) is known to respond to antipsychotic drugs, and (2) for whom alternative, equally effective, but potentially less harmful treatments are not available or appropriate. In patients who do require chronic treatment, the smallest dose and the shortest duration of treatment producing a satisfactory clinical response should be sought. The need for continued treatment should be reassessed periodically.
If signs and symptoms of tardive dyskinesia appear in a patient on iloperidone tablets, drug discontinuation should be considered. However, some patients may require treatment with iloperidone tablets despite the presence of the syndrome.
5.6 Metabolic Changes
Atypical antipsychotic drugs have been associated with metabolic changes that may increase cardiovascular/cerebrovascular risk. These metabolic changes include hyperglycemia, dyslipidemia, and body weight gain. While all atypical antipsychotic drugs have been shown to produce some metabolic changes, each drug in the class has its own specific risk profile.
Hyperglycemia and Diabetes Mellitus
Hyperglycemia, in some cases extreme and associated with ketoacidosis or hyperosmolar coma or death, has been reported in patients treated with atypical antipsychotics including iloperidone tablets. Assessment of the relationship between atypical antipsychotic use and glucose abnormalities is complicated by the possibility of an increased background risk of diabetes mellitus in patients with schizophrenia and the increasing incidence of diabetes mellitus in the general population. Given these confounders, the relationship between atypical antipsychotic use and hyperglycemia-related adverse events is not completely understood. However, epidemiological studies suggest an increased risk of hyperglycemia-related adverse events in patients treated with the atypical antipsychotics included in these studies.
Patients with an established diagnosis of diabetes mellitus who are started on atypical antipsychotics should be monitored regularly for worsening of glucose control. Patients with risk factors for diabetes mellitus (e.g., obesity, family history of diabetes) who are starting treatment with atypical antipsychotics should undergo fasting blood glucose testing at the beginning of treatment and periodically during treatment. Any patient treated with atypical antipsychotics should be monitored for symptoms of hyperglycemia including polydipsia, polyuria, polyphagia, and weakness. Patients who develop symptoms of hyperglycemia during treatment with atypical antipsychotics should undergo fasting blood glucose testing. In some cases, hyperglycemia has resolved when the atypical antipsychotic was discontinued; however, some patients required continuation of antidiabetic treatment despite discontinuation of the suspect drug.
Data from a 4-week, fixed-dose study in adult subjects with schizophrenia, in which fasting blood samples were drawn, are presented in Table 1.
| Placebo | Iloperidone Tablets 24 mg/day |
|
|---|---|---|
| Mean Change from Baseline (mg/dL) | ||
| n=114 | n=228 | |
| Serum Glucose Change from Baseline | -0.5 | 6.6 |
| Proportion of Patients with Shifts | ||
| Serum Glucose Normal to High
(<100 mg/dL to ≥126 mg/dL) | 2.5% | 10.7% |
| (2/80) | (18/169) | |
Pooled analyses of glucose data from clinical studies including longer term trials are shown in Table 2.
| Mean Change from Baseline (mg/dL) | |||
|---|---|---|---|
| 3 months to 6 months | 6 months to 12 months | >12 months | |
| Iloperidone tablets 10 mg/day to 16 mg/day | 1.8 (N=773) | 5.4 (N=723) | 5.4 (N=425) |
| Iloperidone tablets 20 mg/day to 24 mg/day | -3.6 (N=34) | -9 (N=31) | -18 (N=20) |
Dyslipidemia
Undesirable alterations in lipids have been observed in patients treated with atypical antipsychotics.
Data from a placebo-controlled, 4-week, fixed-dose study, in which fasting blood samples were drawn, in adult subjects with schizophrenia are presented in Table 3.
| Placebo | Iloperidone Tablets 24 mg/day |
|
|---|---|---|
| Mean Change from Baseline (mg/dL) | ||
| Cholesterol | n=114 | n=228 |
| Change from baseline | -2.17 | 8.18 |
| LDL | n=109 | n=217 |
| Change from baseline | -1.41 | 9.03 |
| HDL | n=114 | n=228 |
| Change from baseline | -3.35 | 0.55 |
| Triglycerides | n=114 | n=228 |
| Change from baseline | 16.47 | -0.83 |
| Proportion of Patients with Shifts | ||
| Cholesterol | ||
| Normal to High | 1.4% | 3.6% |
| (<200 mg/dL to ≥240 mg/dL) | (1/72) | (5/141) |
| LDL | ||
| Normal to High | 2.4% | 1.1% |
| (<100 mg/dL to ≥160 mg/dL) | (1/42) | (1/90) |
| HDL | ||
| Normal to Low | 23.8% | 12.1% |
| (≥40 mg/dL to <40 mg/dL) | (19/80) | (20/166) |
| Triglycerides | ||
| Normal to High | 8.3% | 10.1% |
| (<150 mg/dL to ≥200 mg/dL) | (6/72) | (15/148) |
Pooled analyses of cholesterol and triglyceride data from clinical studies including longer term trials are shown in Table 4 and Table 5.
| Mean Change from Baseline (mg/dL) | |||
|---|---|---|---|
| 3 months to 6 months | 6 months to 12 months | >12 months | |
| Iloperidone tablets 10 mg/day to16 mg/day | -3.9 (N=783) | -3.9 (N=726) | -7.7 (N=428) |
| Iloperidone tablets 20 mg/day to 24 mg/day | -19.4 (N=34) | -23.2 (N=31) | -19.4 (N=20) |
| Mean Change from Baseline (mg/dL) | |||
|---|---|---|---|
| 3 months to 6 months | 6 months to 12 months | >12 months | |
| Iloperidone tablets 10 mg/day to 16 mg/day | -8.9 (N=783) | -8.9 (N=726) | -17.7 (N=428) |
| Iloperidone tablets 20 mg/day to 24 mg/day | -26.6 (N=34) | -35.4 (N=31) | -17.7 (N=20) |
Weight Gain
Weight gain has been observed with atypical antipsychotic use. Clinical monitoring of weight is recommended.
Across all short- and long-term studies, the overall mean change from baseline at endpoint was 2.1 kg.
Changes in body weight (kg) and the proportion of subjects with ≥7% gain in body weight from 4 placebo-controlled, 4-week or 6-week, fixed- or flexible-dose studies in adult subjects are presented in Table 6.
| Placebo n=576 | Iloperidone Tablets 10 mg/day to 16 mg/day n=481 | Iloperidone Tablets 20 mg/day to 24 mg/day n=391 |
|
|---|---|---|---|
| Weight (kg) Change from Baseline | -0.1 | 2 | 2.7 |
| Weight Gain
≥7% increase from Baseline | 4% | 12% | 18% |
5.7 Seizures
In short-term placebo-controlled trials (4-weeks to 6-weeks), seizures occurred in 0.1% (1/1344) of patients treated with iloperidone tablets compared to 0.3% (2/587) on placebo. As with other antipsychotics, iloperidone tablets should be used cautiously in patients with a history of seizures or with conditions that potentially lower the seizure threshold. Conditions that lower the seizure threshold may be more prevalent in a population of 65 years or older.
5.8 Orthostatic Hypotension and Syncope
Iloperidone tablets can induce orthostatic hypotension associated with dizziness, tachycardia, and syncope. This reflects its alpha1-adrenergic antagonist properties. In double-blind placebo-controlled short-term studies, where the dose was increased slowly, as recommended above, syncope was reported in 0.4% (5/1344) of patients treated with iloperidone tablets, compared with 0.2% (1/587) on placebo. Orthostatic hypotension was reported in 5% of patients given 20 mg/day to 24 mg/day, 3% of patients given 10 mg/day to 16 mg/day, and 1% of patients given placebo. More rapid titration would be expected to increase the rate of orthostatic hypotension and syncope.
Iloperidone tablets should be used with caution in patients with known cardiovascular disease (e.g., heart failure, history of myocardial infarction, ischemia, or conduction abnormalities), cerebrovascular disease, or conditions that predispose the patient to hypotension (dehydration, hypovolemia, and treatment with antihypertensive medications). Monitoring of orthostatic vital signs should be considered in patients who are vulnerable to hypotension.
5.9 Falls
Iloperidone tablets may cause somnolence, postural hypotension, motor and sensory instability, which may lead to falls and, consequently, fractures or other injuries. For patients with diseases, conditions, or medications that could exacerbate these effects, complete fall risk assessments when initiating antipsychotic treatment and recurrently for patients on long-term antipsychotic therapy.
5.10 Leukopenia, Neutropenia and Agranulocytosis
In clinical trial and postmarketing experience, events of leukopenia/neutropenia have been reported temporally related to antipsychotic agents. Agranulocytosis (including fatal cases) has also been reported.
Possible risk factors for leukopenia/neutropenia include preexisting low white blood cell count (WBC) and history of drug induced leukopenia/neutropenia. Patients with a pre-existing low WBC or a history of drug induced leukopenia/neutropenia should have their complete blood count (CBC) monitored frequently during the first few months of therapy and should discontinue iloperidone at the first sign of a decline in WBC in the absence of other causative factors.
Patients with neutropenia should be carefully monitored for fever or other symptoms or signs of infection and treated promptly if such symptoms or signs occur. Patients with severe neutropenia (absolute neutrophil count <1000/mm3) should discontinue iloperidone and have their WBC followed until recovery.
5.11 Hyperprolactinemia
As with other drugs that antagonize dopamine D2 receptors, iloperidone tablets elevate prolactin levels.
Hyperprolactinemia may suppress hypothalamic GnRH, resulting in reduced pituitary gonadotropin secretion. This, in turn, may inhibit reproductive function by impairing gonadalsteroidogenesis in both female and male patients. Galactorrhea, amenorrhea, gynecomastia, and impotence have been reported with prolactin-elevating compounds. Long-standing hyperprolactinemia when associated with hypogonadism may lead to decreased bone density in both female and male patients.
Tissue culture experiments indicate that approximately one-third of human breast cancers are prolactin-dependent in vitro, a factor of potential importance if the prescription of these drugs is contemplated in a patient with previously detected breast cancer. Mammary gland proliferative changes and increases in serum prolactin were seen in mice and rats treated with iloperidone [see Nonclinical Toxicology (13)]. Neither clinical studies nor epidemiologic studies conducted to date have shown an association between chronic administration of this class of drugs and tumorigenesis in humans; the available evidence is considered too limited to be conclusive at this time.
In a short-term placebo-controlled trial (4-weeks), the mean change from baseline to endpoint in plasma prolactin levels for the iloperidone tablets 24 mg/day-treated group was an increase of 2.6 ng/mL compared to a decrease of 6.3 ng/mL in the placebo-group. In this trial, elevated plasma prolactin levels were observed in 26% of adults treated with iloperidone tablets compared to 12% in the placebo group. In the short-term trials, iloperidone was associated with modest levels of prolactin elevation compared to greater prolactin elevations observed with some other antipsychotic agents. In pooled analysis from clinical studies including longer term trials, in 3210 adults treated with iloperidone, gynecomastia was reported in 2 male subjects (0.1%) compared to 0% in placebo-treated patients, and galactorrhea was reported in 8 female subjects (0.2%) compared to 3 female subjects (0.5%) in placebo-treated patients.
5.12 Body Temperature Regulation
Disruption of the body's ability to reduce core body temperature has been attributed to antipsychotic agents. Appropriate care is advised when prescribing iloperidone tablets for patients who will be experiencing conditions which may contribute to an elevation in core body temperature, e.g., exercising strenuously, exposure to extreme heat, receiving concomitant medication with anticholinergic activity, or being subject to dehydration.
5.13 Dysphagia
Esophageal dysmotility and aspiration have been associated with antipsychotic drug use. Aspiration pneumonia is a common cause of morbidity and mortality in elderly patients. Iloperidone tablets and other antipsychotic drugs should be used cautiously in patients at risk for aspiration pneumonia [see Boxed Warning].
5.14 Suicide
The possibility of a suicide attempt is inherent in psychotic illness, and close supervision of high-risk patients should accompany drug therapy. Prescriptions for iloperidone tablets should be written for the smallest quantity of tablets consistent with good patient management in order to reduce the risk of overdose.
5.15 Priapism
Three cases of priapism were reported in the pre-marketing iloperidone tablet program. Drugs with alpha-adrenergic blocking effects have been reported to induce priapism. Iloperidone shares this pharmacologic activity. Severe priapism may require surgical intervention.
5.16 Potential for Cognitive and Motor Impairment
Iloperidone tablets, like other antipsychotics, has the potential to impair judgment, thinking or motor skills. In short-term, placebo-controlled trials, somnolence (including sedation) was reported in 11.9% (104/874) of adult patients treated with iloperidone tablets at doses of 10 mg/day or greater versus 5.3% (31/587) treated with placebo. Patients should be cautioned about operating hazardous machinery, including automobiles, until they are reasonably certain that therapy with iloperidone tablets does not affect them adversely.
6 ADVERSE REACTIONS
6.1 Clinical Studies Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trial of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice. The information below is derived from a clinical trial database for iloperidone tablets consisting of 3229 patients exposed to iloperidone tablets at doses of 10 mg/day or greater, for the treatment of schizophrenia. Of these, 999 received iloperidone tablets for at least 6 months, with 657 exposed to iloperidone tablets for at least 12 months. All of these patients who received iloperidone tablets were participating in multiple-dose clinical trials. The conditions and duration of treatment with iloperidone tablets varied greatly and included (in overlapping categories), open-label and double-blind phases of studies, inpatients and outpatients, fixed-dose and flexible-dose studies, and short-term and longer-term exposure.
The information presented in these sections was derived from pooled data from 4 placebo-controlled, 4-week or 6-week, fixed- or flexible-dose studies in patients who received iloperidone tablets at daily doses within a range of 10 mg to 24 mg (n=874).
Adverse Reactions Occurring at an Incidence of 2% or More among Iloperidone Tablet-Treated Patients and More Frequent than Placebo
Table 7 enumerates the pooled incidences of adverse reactions that were spontaneously reported in four placebo-controlled, 4-week or 6-week, fixed- or flexible-dose studies, listing those reactions that occurred in 2% or more of patients treated with iloperidone tablets in any of the dose groups, and for which the incidence in iloperidone tablet-treated patients in any dose group was greater than the incidence in patients treated with placebo.
| Body System or Organ Class | Placebo | Iloperidone Tablets 10 mg/day to 16 mg/day | Iloperidone Tablets 20 mg/day to 24 mg/day |
|---|---|---|---|
| % | % | % | |
| Dictionary-derived Term | (N=587) | (N=483) | (N=391) |
|
|||
| Body as a Whole | |||
| Arthralgia | 2 | 3 | 3 |
| Fatigue | 3 | 4 | 6 |
| Musculoskeletal Stiffness | 1 | 1 | 3 |
| Weight Increased | 1 | 1 | 9 |
| Cardiac Disorders | |||
| Tachycardia | 1 | 3 | 12 |
| Eye Disorders | |||
| Vision Blurred | 2 | 3 | 1 |
| Gastrointestinal Disorders | |||
| Nausea | 8 | 7 | 10 |
| Dry Mouth | 1 | 8 | 10 |
| Diarrhea | 4 | 5 | 7 |
| Abdominal Discomfort | 1 | 1 | 3 |
| Infections | |||
| Nasopharyngitis | 3 | 4 | 3 |
| Upper Respiratory Tract Infection | 1 | 2 | 3 |
| Nervous System Disorders | |||
| Dizziness | 7 | 10 | 20 |
| Somnolence | 5 | 9 | 15 |
| Extrapyramidal Disorder | 4 | 5 | 4 |
| Tremor | 2 | 3 | 3 |
| Lethargy | 1 | 3 | 1 |
| Reproductive System | |||
| Ejaculation Failure | <1 | 2 | 2 |
| Respiratory | |||
| Nasal Congestion | 2 | 5 | 8 |
| Dyspnea | <1 | 2 | 2 |
| Skin | |||
| Rash | 2 | 3 | 2 |
| Vascular Disorders | |||
| Orthostatic Hypotension | 1 | 3 | 5 |
| Hypotension | <1 | <1 | 3 |
Dose-Related Adverse Reactions in Clinical Trials
Based on the pooled data from 4 placebo-controlled, 4-week or 6-week, fixed- or flexible-dose studies, adverse reactions that occurred with a greater than 2% incidence in the patients treated with iloperidone tablets, and for which the incidence in patients treated with iloperidone tablets 20 mg/day to 24 mg/day were twice than the incidence in patients treated with iloperidone tablets 10 mg/day to 16 mg/day were: abdominal discomfort, dizziness, hypotension, musculoskeletal stiffness, tachycardia, and weight increased.
Common and Drug-Related Adverse Reactions in Clinical Trials
Based on the pooled data from 4 placebo-controlled, 4-week or 6-week, fixed- or flexible-dose studies, the following adverse reactions occurred in ≥5% incidence in the patients treated with iloperidone tablets and at least twice the placebo rate for at least 1 dose: dizziness, dry mouth, fatigue, nasal congestion, somnolence, tachycardia, orthostatic hypotension, and weight increased. Dizziness, tachycardia, and weight increased were at least twice as common on 20 mg/day to 24 mg/day as on 10 mg/day to 16 mg/day.
Extrapyramidal Symptoms (EPS) in Clinical Trials
Pooled data from the 4 placebo-controlled, 4-week or 6-week, fixed- or flexible-dose studies provided information regarding EPS. Adverse event data collected from those trials showed the following rates of EPS-related adverse events as shown in Table 8.
| Adverse Event Term | Placebo (%) (N=587) | Iloperidone Tablets 10 mg/day to 16 mg/day (%) (N=483) | Iloperidone Tablets 20 mg/day to 24 mg/day (%) (N=391) |
|---|---|---|---|
| All EPS events | 11.6 | 13.5 | 15.1 |
| Akathisia | 2.7 | 1.7 | 2.3 |
| Bradykinesia | 0 | 0.6 | 0.5 |
| Dyskinesia | 1.5 | 1.7 | 1 |
| Dystonia | 0.7 | 1 | 0.8 |
| Parkinsonism | 0 | 0.2 | 0.3 |
| Tremor | 1.9 | 2.5 | 3.1 |
Adverse Reactions Associated with Discontinuation of Treatment in Clinical Trials
Based on the pooled data from 4 placebo-controlled, 4-week or 6-week, fixed- or flexible-dose studies, there was no difference in the incidence of discontinuation due to adverse events between iloperidone tablet-treated (5%) and placebo-treated (5%) patients. The types of adverse events that led to discontinuation were similar for the iloperidone tablet- and placebo-treated patients.
Demographic Differences in Adverse Reactions in Clinical Trials
An examination of population subgroups in the 4 placebo-controlled, 4-week or 6-week, fixed- or flexible-dose studies did not reveal any evidence of differences in safety on the basis of age, gender or race.
Laboratory Test Abnormalities in Clinical Trials
There were no differences between iloperidone tablets and placebo in the incidence of discontinuation due to changes in hematology, urinalysis, or serum chemistry.
In short-term placebo-controlled trials (4-weeks to 6-weeks), there were 1% (13/1342) iloperidone-treated patients with hematocrit at least one time below the extended normal range during post-randomization treatment, compared to 0.3% (2/585) on placebo. The extended normal range for lowered hematocrit was defined in each of these trials as the value 15% below the normal range for the centralized laboratory that was used in the trial.
Other Reactions During the Pre-marketing Evaluation of Iloperidone Tablets
The following is a list of MedDRA terms that reflect adverse reactions in patients treated with iloperidone tablets at multiple doses ≥ 4 mg/day during any phase of a trial with the database of 3210 iloperidone tablet-treated patients. All reported reactions are included except those already listed in Table 7, or other parts of the Adverse Reactions (6), those considered in the Warnings and Precautions (5), those reaction terms which were so general as to be uninformative, reactions reported in fewer than 3 patients and which were neither serious nor life-threatening, reactions that are otherwise common as background reactions, and reactions considered unlikely to be drug related.
Reactions are further categorized by MedDRA system organ class and listed in order of decreasing frequency according to the following definitions: frequent adverse events are those occurring in at least 1/100 patients (only those not listed in Table 7 appear in this listing); infrequent adverse reactions are those occurring in 1/100 to 1/1000 patients; rare events are those occurring in fewer than 1/1000 patients.
Blood and Lymphatic Disorders: Infrequent – anemia, iron deficiency anemia; Rare – leukopenia
Cardiac Disorders: Frequent – palpitations; Rare – arrhythmia, atrioventricular block first degree, cardiac failure (including congestive and acute)
Ear and Labyrinth Disorders: Infrequent – vertigo, tinnitus
Endocrine Disorders: Infrequent – hypothyroidism
Eye Disorders: Frequent – conjunctivitis (including allergic); Infrequent – dry eye, blepharitis, eyelid edema, eye swelling, lenticular opacities, cataract, hyperemia (including conjunctival)
Gastrointestinal Disorders: Infrequent – gastritis, salivary hypersecretion, fecal incontinence, mouth ulceration; Rare – aphthous stomatitis, duodenal ulcer, hiatus hernia, hyperchlorhydria, lip ulceration, reflux esophagitis, stomatitis
General Disorders and Administrative Site Conditions: Infrequent – edema (general, pitting, due to cardiac disease), difficulty in walking, thirst; Rare – hyperthermia
Hepatobiliary Disorders: Infrequent – cholelithiasis
Investigations: Frequent – weight decreased; Infrequent – hemoglobin decreased, neutrophil count increased, hematocrit decreased
Metabolism and Nutrition Disorders: Infrequent – increased appetite, dehydration, hypokalemia, fluid retention
Musculoskeletal and Connective Tissue Disorders: Frequent – myalgia, muscle spasms; Rare – torticollis
Nervous System Disorders: Infrequent – paresthesia, psychomotor hyperactivity, restlessness, amnesia, nystagmus; Rare – restless legs syndrome
Psychiatric Disorders: Frequent – restlessness, aggression, delusion; Infrequent – hostility, libido decreased, paranoia, anorgasmia, confusional state, mania, catatonia, mood swings, panic attack, obsessive-compulsive disorder, bulimia nervosa, delirium, polydipsia psychogenic, impulse-control disorder, major depression
Renal and Urinary Disorders: Frequent – urinary incontinence; Infrequent – dysuria, pollakiuria, enuresis, nephrolithiasis; Rare – urinary retention, renal failure acute
Reproductive System and Breast Disorders: Frequent – erectile dysfunction; Infrequent – testicular pain, amenorrhea, breast pain; Rare – menstruation irregular, gynecomastia, menorrhagia, metrorrhagia, postmenopausal hemorrhage, prostatitis.
Respiratory, Thoracic and Mediastinal Disorders: Infrequent – epistaxis, asthma, rhinorrhea, sinus congestion, nasal dryness; Rare – dry throat, sleep apnea syndrome, dyspnea exertional
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of iloperidone tablets: retrograde ejaculation and hypersensitivity reactions (including anaphylaxis; angioedema; throat tightness; oropharyngeal swelling; swelling of the face, lips, mouth, and tongue; urticaria; rash; and pruritus). Because these reactions were reported voluntarily from a population of uncertain size, it is not possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
7 DRUG INTERACTIONS
Given the primary CNS effects of iloperidone tablets, caution should be used when it is taken in combination with other centrally acting drugs and alcohol. Due to its - alpha1-adrenergic receptor antagonism, iloperidone tablets have the potential to enhance the effect of certain antihypertensive agents.
7.1 Potential for Other Drugs to Affect Iloperidone Tablets
Iloperidone is not a substrate for CYP1A1, CYP1A2, CYP2A6, CYP2B6, CYP2C8, CYP2C9, CYP2C19, or CYP2E1 enzymes. This suggests that an interaction of iloperidone with inhibitors or inducers of these enzymes, or other factors, like smoking, is unlikely.
Both CYP3A4 and CYP2D6 are responsible for iloperidone metabolism. Inhibitors of CYP3A4 (e.g., ketoconazole) or CYP2D6 (e.g., fluoxetine, paroxetine) can inhibit iloperidone elimination and cause increased blood levels.
Ketoconazole: Coadministration of ketoconazole (200 mg twice daily for 4 days), a potent inhibitor of CYP3A4, with a 3 mg single dose of iloperidone to 19 healthy volunteers, ages 18 years to 45 years, increased the area under the curve (AUC) of iloperidone and its metabolites P88 and P95 by 57%, 55% and 35%, respectively. Iloperidone doses should be reduced by about one-half when administered with ketoconazole or other strong inhibitors of CYP3A4 (e.g., itraconazole). Weaker inhibitors (e.g., erythromycin, grapefruit juice) have not been studied. When the CYP3A4 inhibitor is withdrawn from the combination therapy, the iloperidone dose should be returned to the previous level.
Fluoxetine: Coadministration of fluoxetine (20 mg twice daily for 21 days), a potent inhibitor of CYP2D6, with a single 3 mg dose of iloperidone to 23 healthy volunteers, ages 29 years to 44 years, who were classified as CYP2D6 extensive metabolizers, increased the AUC of iloperidone and its metabolite P88, by about 2-fold to 3-fold, and decreased the AUC of its metabolite P95 by one-half. Iloperidone doses should be reduced by one-half when administered with fluoxetine. When fluoxetine is withdrawn from the combination therapy, the iloperidone dose should be returned to the previous level. Other strong inhibitors of CYP2D6 would be expected to have similar effects and would need appropriate dose reductions. When the CYP2D6 inhibitor is withdrawn from the combination therapy, iloperidone dose could then be increased to the previous level.
Paroxetine: Coadministration of paroxetine (20 mg/day for 5 days to 8 days), a potent inhibitor of CYP2D6, with multiple doses of iloperidone (8 mg or 12 mg twice daily) to patients with schizophrenia ages 18 years to 65 years resulted in increased mean steady-state peak concentrations of iloperidone and its metabolite P88, by about 1.6 fold, and decreased mean steady-state peak concentrations of its metabolite P95 by one-half. Iloperidone doses should be reduced by one-half when administered with paroxetine. When paroxetine is withdrawn from the combination therapy, the iloperidone dose should be returned to the previous level. Other strong inhibitors of CYP2D6 would be expected to have similar effects and would need appropriate dose reductions. When the CYP2D6 inhibitor is withdrawn from the combination therapy, iloperidone dose could then be increased to previous levels.
Paroxetine and Ketoconazole: Coadministration of paroxetine (20 mg once daily for 10 days), a CYP2D6 inhibitor, and ketoconazole (200 mg twice daily) with multiple doses of iloperidone (8 mg or 12 mg twice daily) to patients with schizophrenia ages 18 years to 65 years resulted in a 1.4 fold increase in steady-state concentrations of iloperidone and its metabolite P88 and a 1.4 fold decrease in the P95 in the presence of paroxetine. So giving iloperidone with inhibitors of both of its metabolic pathways did not add to the effect of either inhibitor given alone. Iloperidone doses should therefore be reduced by about one-half if administered concomitantly with both a CYP2D6 and CYP3A4 inhibitor.
7.2 Potential for Iloperidone Tablets to Affect Other Drugs
In vitro studies in human liver microsomes showed that iloperidone does not substantially inhibit the metabolism of drugs metabolized by the following cytochrome P450 isozymes: CYP1A1, CYP1A2, CYP2A6, CYP2B6, CYP2C8, CYP2C9, or CYP2E1. Furthermore, in vitro studies in human liver microsomes showed that iloperidone does not have enzyme inducing properties, specifically for the following cytochrome P450 isozymes: CYP1A2, CYP2C8, CYP2C9, CYP2C19, CYP3A4 and CYP3A5.
Dextromethorphan: A study in healthy volunteers showed that changes in the pharmacokinetics of dextromethorphan (80 mg dose) when a 3 mg dose of iloperidone was coadministered resulted in a 17% increase in total exposure and a 26% increase in the maximum plasma concentrations Cmax of dextromethorphan. Thus, an interaction between iloperidone and other CYP2D6 substrates is unlikely.
Fluoxetine: A single 3 mg dose of iloperidone had no effect on the pharmacokinetics of fluoxetine (20 mg twice daily).
Midazolam (a sensitive CYP3A4 substrate): A study in patients with schizophrenia showed a less than 50% increase in midazolam total exposure at iloperidone steady state (14 days of oral dosing at up to 10 mg iloperidone twice daily) and no effect on midazolam Cmax. Thus, an interaction between iloperidone and other CYP3A4 substrates is unlikely.
7.3 Drugs that Prolong the QT Interval
Iloperidone tablets should not be used with any other drugs that prolong the QT interval [see Warnings and Precautions (5.3)].
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Exposure Registry
There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to iloperidone during pregnancy. For more information contact the National Pregnancy Registry for Atypical Antipsychotics at 1-866-961-2388 or visit http://womensmentalhealth.org/clinical-and-research-programs/pregnancyregistry/.
Risk Summary
Neonates whose mothers are exposed to antipsychotic drugs, including iloperidone tablets, during the third trimester of pregnancy are at risk for extrapyramidal and/or withdrawal symptoms following delivery [see Clinical Considerations]. The limited available data with iloperidone tablets in pregnant women are not sufficient to inform a drug-associated risk for major birth defects and miscarriage. Iloperidone was not teratogenic when administered orally to pregnant rats during organogenesis at doses up to 26 times the maximum recommended human dose of 24 mg/day on mg/m2 basis. However, it prolonged the duration of pregnancy and parturition, increased still births, early intrauterine deaths, increased incidence of developmental delays, and decreased post-partum pup survival. Iloperidone was not teratogenic when administered orally to pregnant rabbits during organogenesis at doses up to 20-times the MRHD on mg/m2 basis. However, it increased early intrauterine deaths and decreased fetal viability at term at the highest dose which was also a maternally toxic dose [see Data].
The background risk of major birth defects and miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Clinical Considerations
Fetal/Neonatal Adverse Reactions
Extrapyramidal and/or withdrawal symptoms, including agitation, hypertonia, hypotonia, tremor, somnolence, respiratory distress and feeding disorder have been reported in neonates whose mothers were exposed to antipsychotic drugs during the third trimester of pregnancy. These symptoms have varied in severity. Some neonates recovered within hours or days without specific treatment; others required prolonged hospitalization. Monitor neonates for extrapyramidal and/or withdrawal symptoms and manage symptoms appropriately.
Data
Animal Data
In an embryo-fetal development study, pregnant rats were given 4 mg/kg/day, 16 mg/kg/day, or 64 mg/kg/day (1.6 times, 6.5 times, and 26 times the maximum recommended human dose (MRHD) of 24 mg/day on a mg/m2 basis) of iloperidone orally during the period of organogenesis. The highest dose caused increased early intrauterine deaths, decreased fetal weight and length, decreased fetal skeletal ossification, and an increased incidence of minor fetal skeletal anomalies and variations; this dose also caused decreased maternal food consumption and weight gain.
In an embryo-fetal development study, pregnant rabbits were given 4 mg/kg/day, 10 mg/kg/day, or 25 mg/kg/day (3 times, 8 times, and 20 times the MRHD on a mg/m2 basis) of iloperidone during the period of organogenesis. The highest dose caused increased early intrauterine deaths and decreased fetal viability at term; this dose also caused maternal toxicity.
In additional studies in which rats were given iloperidone at doses similar to the above beginning from either pre-conception or from day 17 of gestation and continuing through weaning, adverse reproductive effects included prolonged pregnancy and parturition, increased stillbirth rates, increased incidence of fetal visceral variations, decreased fetal and pup weights, and decreased post-partum pup survival. There were no drug effects on the neurobehavioral or reproductive development of the surviving pups. No-effect doses ranged from 4 mg/kg to 12 mg/kg except for the increase in stillbirth rates which occurred at the lowest dose tested of 4 mg/kg, which is 1.6 times the MRHD on a mg/m2 basis. Maternal toxicity was seen at the higher doses in these studies.
The iloperidone metabolite P95, which is a major circulating metabolite of iloperidone in humans but is not present in significant amounts in rats, was given to pregnant rats during the period of organogenesis at oral doses of 20 mg/kg/day, 80 mg/kg/day, or 200 mg/kg/day. No teratogenic effects were seen. Delayed skeletal ossification occurred at all doses. No significant maternal toxicity was produced. Plasma levels of P95 (AUC) at the highest dose tested were 2 times those in humans receiving the MRHD of iloperidone.
8.2 Lactation
Risk Summary
There is no information regarding the presence of iloperidone or its metabolites in human milk, the effects of iloperidone on a breastfed child, nor the effects of iloperidone on human milk production. Iloperidone is present in rat milk [see Data]. Because of the potential for serious adverse reactions in breastfed infants, advise a woman not to breastfeed during treatment with iloperidone tablets.
Data
The transfer of radioactivity into the milk of lactating rats was investigated following a single dose of [14C] iloperidone at 5 mg/kg. The concentration of radioactivity in milk at 4 hours post-dose was near 10-fold greater than that in plasma at the same time. However, by 24 hours after dosing, concentrations of radioactivity in milk had fallen to values slightly lower than plasma. The metabolic profile in milk was qualitatively similar to that in plasma.
8.3 Pediatric Use
Safety and effectiveness in pediatric and adolescent patients have not been established.
8.4 Geriatric Use
Clinical Studies of iloperidone tablets in the treatment of schizophrenia did not include sufficient numbers of patients aged 65 years and over to determine whether or not they respond differently than younger adult patients. Of the 3210 patients treated with iloperidone tablets in premarketing trials, 25 (0.5%) were ≥65 years old and there were no patients ≥75 years old.
Elderly patients with dementia-related psychosis treated with iloperidone tablets are at an increased risk of death compared to placebo. Iloperidone tablets are not approved for the treatment of patients with dementia-related psychosis [see Boxed Warning and Warnings and Precautions (5.1, 5.2)].
8.5 Renal Impairment
Because iloperidone is highly metabolized, with less than 1% of the drug excreted unchanged, renal impairment alone is unlikely to have a significant impact on the pharmacokinetics of iloperidone. Renal impairment (creatinine clearance <30 mL/min) had minimal effect on Cmax of iloperidone (given in a single dose of 3 mg) and its metabolites P88 and P95 in any of the 3 analytes measured. AUC0–∞ was increased by 24%, decreased by 6%, and increased by 52% for iloperidone, P88 and P95, respectively, in subjects with renal impairment.
8.6 Hepatic Impairment
No dose adjustment to iloperidone tablets is needed in patients with mild hepatic impairment. Patients with moderate hepatic impairment may require dose reduction. Iloperidone tablets are not recommended for patients with severe hepatic impairment [see Dosage and Administration (2.2)].
In adult subjects with mild hepatic impairment no relevant difference in pharmacokinetics of iloperidone, P88 or P95 (total or unbound) was observed compared to healthy adult controls. In subjects with moderate hepatic impairment a higher (2-fold) and more variable free exposure to the active metabolites P88 was observed compared to healthy controls, whereas exposure to iloperidone and P95 was generally similar (less than 50% change compared to control). Since a study in severe liver impaired subjects has not been conducted, iloperidone tablets are not recommended for patients with severe hepatic impairment.
9 DRUG ABUSE AND DEPENDENCE
9.2 Abuse
Iloperidone has not been systematically studied in animals or humans for its potential for abuse, tolerance, or physical dependence. While the clinical trials did not reveal any tendency for drug-seeking behavior, these observations were not systematic and it is not possible to predict on the basis of this experience the extent to which a CNS active drug, iloperidone, will be misused, diverted, and/or abused once marketed. Consequently, patients should be evaluated carefully for a history of drug abuse, and such patients should be observed closely for signs of iloperidone tablet misuse or abuse (e.g., development of tolerance, increases in dose, drug-seeking behavior).
10 OVERDOSAGE
10.1 Human Experience
In pre-marketing trials involving over 3210 patients, accidental or intentional overdose of iloperidone tablets was documented in 8 patients ranging from 48 mg to 576 mg taken at once and 292 mg taken over a 3-day period. No fatalities were reported from these cases. The largest confirmed single ingestion of iloperidone was 576 mg; no adverse physical effects were noted for this patient. The next largest confirmed ingestion of iloperidone was 438 mg over a 4-day period; extrapyramidal symptoms and a QTc interval of 507 msec were reported for this patient with no cardiac sequelae. This patient resumed iloperidone tablet treatment for an additional 11 months. In general, reported signs and symptoms were those resulting from an exaggeration of the known pharmacological effects (e.g., drowsiness and sedation, tachycardia and hypotension) of iloperidone.
10.2 Management of Overdose
There is no specific antidote for iloperidone tablets. Therefore appropriate supportive measures should be instituted. In case of acute overdose, the physician should establish and maintain an airway and ensure adequate oxygenation and ventilation. Gastric lavage (after intubation, if patient is unconscious) and administration of activated charcoal together with a laxative should be considered. The possibility of obtundation, seizures or dystonic reaction of the head and neck following overdose may create a risk of aspiration with induced emesis. Cardiovascular monitoring should commence immediately and should include continuous ECG monitoring to detect possible arrhythmias. If antiarrhythmic therapy is administered, disopyramide, procainamide and quinidine should not be used, as they have the potential for QT-prolonging effects that might be additive to those of iloperidone. Similarly, it is reasonable to expect that the alpha-blocking properties of bretylium might be additive to those of iloperidone, resulting in problematic hypotension. Hypotension and circulatory collapse should be treated with appropriate measures such as intravenous fluids or sympathomimetic agents (epinephrine and dopamine should not be used, since beta stimulation may worsen hypotension in the setting of iloperidone-induced alpha blockade). In cases of severe extrapyramidal symptoms, anticholinergic medication should be administered. Close medical supervision should continue until the patient recovers.
11 DESCRIPTION
Iloperidone is an atypical antipsychotic belonging to the chemical class of piperidinyl-benzisoxazole derivatives. Its chemical name is 4'-[3-[4-(6-Fluoro-1,2-benzisoxazol-3-yl)piperidino]propoxy]-3'-methoxyacetophenone. Its molecular formula is C24H27FN2O4 and its molecular weight is 426.48.
The structural formula is:
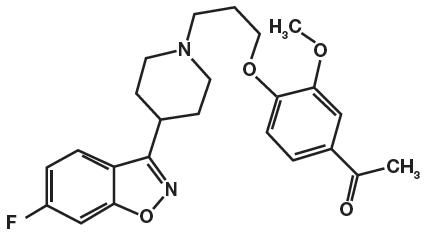
Iloperidone is a white to off-white finely crystalline powder. It is practically insoluble in water, very slightly soluble in 0.1 N HCl and freely soluble in chloroform, ethanol, methanol, and acetonitrile.
Iloperidone tablets are intended for oral administration only. Each uncoated tablet contains 1 mg, 2 mg, 4 mg, 6 mg, 8 mg, 10 mg, or 12 mg of iloperidone. Inactive ingredients are: colloidal silicon dioxide, crospovidone, hypromellose, lactose monohydrate, magnesium stearate, microcrystalline cellulose, and purified water (removed during processing). The oval (1 mg) white tablets are engraved with "T" on one side and on the other side the tablet strength. The round (2 mg, 4 mg, 6 mg, 8 mg, 10 mg or 12 mg) white tablets are engraved with "T" on one side and on the other side the tablet strength.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The mechanism of action of iloperidone in schizophrenia is unknown. However the efficacy of iloperidone could be mediated through a combination of dopamine type 2 (D2) and serotonin type 2 (5-HT2) antagonism. Iloperidone forms an active metabolite, P88, that has an in vitro receptor binding profile similar to the parent drug.
12.2 Pharmacodynamics
Iloperidone acts as an antagonist with high (nM) affinity binding to serotonin 5-HT2A dopamine D2 and D3 receptors, and norepinephrine NEα1 receptors (Ki values of 5.6 nM, 6.3 nM, 7.1 nM, and 0.36 nM, respectively). Iloperidone has moderate affinity for dopamine D4, and serotonin 5-HT6 and 5-HT7 receptors (Ki values of 25 nM, 43 nM, and 22 nM respectively), and low affinity for the serotonin 5-HT1A, dopamine D1, and histamine H1 receptors (Ki values of 168 nM, 216 nM and 437 nM, respectively). Iloperidone has no appreciable affinity (Ki>1000 nM) for cholinergic muscarinic receptors. The affinity of the iloperidone metabolite P88 is generally equal to or less than that of the parent compound, while the metabolite P95 only shows affinity for 5-HT2A (Ki value of 3.91) and the NEα1A, NEα1B, NEα1D, and NEα2C receptors (Ki values of 4.7 nM, 2.7 nM, 8.8 nM and 4.7 nM respectively).
12.3 Pharmacokinetics
The observed mean elimination half-lives for iloperidone, P88 and P95 in CYP2D6 extensive metabolizers (EM) are 18 hours, 26 hours and 23 hours, respectively, and in poor metabolizers (PM) are 33 hours, 37 hours and 31 hours, respectively. Steady-state concentrations are attained within 3 days to 4 days of dosing. Iloperidone accumulation is predictable from single-dose pharmacokinetics. The pharmacokinetics of iloperidone is more than dose proportional. Elimination of iloperidone is mainly through hepatic metabolism involving 2 P450 isozymes, CYP2D6 and CYP3A4.
Absorption: Iloperidone is well absorbed after administration of the tablet with peak plasma concentrations occurring within 2 hours to 4 hours; while the relative bioavailability of the tablet formulation compared to oral solution is 96%. Administration of iloperidone with a standard high-fat meal did not significantly affect the Cmax or AUC of iloperidone, P88, or P95, but delayed Tmax by 1 hour for iloperidone, 2 hours for P88 and 6 hours for P95. Iloperidone tablets can be administered without regard to meals.
Distribution: Iloperidone has an apparent clearance (clearance / bioavailability) of 47 L/h to 102 L/h, with an apparent volume of distribution of 1340 L to 2800 L. At therapeutic concentrations, the unbound fraction of iloperidone in plasma is ~3% and of each metabolite (P88 and P95) it is ~8%.
Metabolism and Elimination: Iloperidone is metabolized primarily by 3 biotransformation pathways: carbonyl reduction, hydroxylation (mediated by CYP2D6) and O-demethylation (mediated by CYP3A4). There are 2 predominant iloperidone metabolites, P95 and P88. The iloperidone metabolite P95 represents 47.9% of the AUC of iloperidone and its metabolites in plasma at steady-state for extensive metabolizers (EM) and 25% for poor metabolizers (PM). The active metabolite P88 accounts for 19.5% and 34% of total plasma exposure in EM and PM, respectively.
Approximately 7% to 10% of Caucasians and 3% to 8% of Black/African Americans lack the capacity to metabolize CYP2D6 substrates and are classified as poor metabolizers (PM), whereas the rest are intermediate, extensive or ultrarapid metabolizers. Coadministration of iloperidone tablets with known strong inhibitors of CYP2D6 like fluoxetine results in a 2.3-fold increase in iloperidone plasma exposure, and therefore one-half of the iloperidone tablet dose should be administered.
Similarly, PMs of CYP2D6 have higher exposure to iloperidone compared with EMs and PMs should have their dose reduced by one-half. Laboratory tests are available to identify CYP2D6 PMs.
The bulk of the radioactive materials were recovered in the urine (mean 58.2% and 45.1% in EM and PM, respectively), with feces accounting for 19.9% (EM) to 22.1% (PM) of the dosed radioactivity.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis: Lifetime carcinogenicity studies were conducted in CD-1 mice and Sprague Dawley rats. Iloperidone was administered orally at doses of 2.5 mg/kg/day, 5 mg/kg/day and 10 mg/kg/day to CD-1 mice and 4 mg/kg/day, 8 mg/kg/day and 16 mg/kg/day to Sprague Dawley rats (0.5 times, 1 time and 2 times and 1.6 times, 3.2 times and 6.5 times, respectively, the MRHD of 24 mg/day on a mg/m2 basis). There was an increased incidence of malignant mammary gland tumors in female mice treated with the lowest dose (2.5 mg/kg/day) only. There were no treatment-related increases in neoplasia in rats.
The carcinogenic potential of the iloperidone metabolite P95, which is a major circulating metabolite of iloperidone in humans but is not present at significant amounts in mice or rats, was assessed in a lifetime carcinogenicity study in Wistar rats at oral doses of 25 mg/kg/day, 75 mg/kg/day and 200 mg/kg/day in males and 50 mg/kg/day, 150 mg/kg/day and 250 (reduced from 400) mg/kg/day in females. Drug-related neoplastic changes occurred in males, in the pituitary gland (pars distalis adenoma) at all doses and in the pancreas (islet cell adenoma) at the high dose. Plasma levels of P95 (AUC) in males at the tested doses (25 mg/kg/day, 75 mg/kg/day, and 200 mg/kg/day) were approximately 0.4 times, 3 times, and 23 times, respectively, the human exposure to P95 at the MRHD of iloperidone.
Mutagenesis: Iloperidone was negative in the Ames test and in the in vivo mouse bone marrow and rat liver micronucleus tests. Iloperidone induced chromosomal aberrations in Chinese Hamster Ovary (CHO) cells in vitro at concentrations which also caused some cytotoxicity.
The iloperidone metabolite P95 was negative in the Ames test, the V79 chromosome aberration test, and an in vivo mouse bone marrow micronucleus test.
14 CLINICAL STUDIES
The efficacy of iloperidone tablets in the treatment of schizophrenia was supported by 2 placebo- and active-controlled short-term (4-week and 6-week) trials and one long-term placebo-controlled randomized withdrawal trial. All trials enrolled patients who met the DSM-III/IV criteria for schizophrenia.
Three instruments were used for assessing psychiatric signs and symptoms in these studies. The Positive and Negative Syndrome Scale (PANSS) and Brief Psychiatric Rating Scale (BPRS) are both multi-item inventories of general psychopathology usually used to evaluate the effects of drug treatment in schizophrenia. The Clinical Global Impression (CGI) assessment reflects the impression of a skilled observer, fully familiar with the manifestations of schizophrenia, about the overall clinical state of the patient.
A 6-week, placebo-controlled trial (n=706) involved 2 flexible dose ranges of iloperidone (12 mg/day to 16 mg/day or 20 mg/day to 24 mg/day) compared to placebo and an active control (risperidone). For the 12 mg/day to 16 mg/day group, the titration schedule of iloperidone tablets was 1 mg twice daily on Days 1 and 2, 2 mg twice daily on Days 3 and 4, 4 mg twice daily on Days 5 and 6, and 6 mg twice daily on Day 7. For the 20 mg/day to 24 mg/day group, the titration schedule of iloperidone tablets was 1 mg twice daily on Day 1, 2 mg twice daily on Day 2, 4 mg twice daily on Day 3, 6 mg twice daily on Days 4 and 5, 8 mg twice daily on Day 6, and 10 mg twice daily on Day 7. The primary endpoint was change from baseline on the BPRS total score at the end of treatment (Day 42). Both the 12 mg/day to 16 mg/day and the 20 mg/day to 24 mg/day dose ranges of iloperidone tablets were superior to placebo on the BPRS total score. The active control antipsychotic drug appeared to be superior to iloperidone tablets in this trial within the first 2 weeks, a finding that may in part be explained by the more rapid titration that was possible for that drug. In patients in this study who remained on treatment for at least 2 weeks, iloperidone appeared to have had comparable efficacy to the active control.
A 4-week, placebo-controlled trial (n=604) involved one fixed dose of iloperidone (24 mg/day) compared to placebo and an active control (ziprasidone). The titration schedule for this study was similar to that for the 6-week study. This study involved titration of iloperidone starting at 1 mg twice daily on Day 1 and increasing to 2 mg, 4 mg, 6 mg, 8 mg, 10 mg and 12 mg twice daily on Days 2, 3, 4, 5, 6, and 7. The primary endpoint was change from baseline on the PANSS total score at the end of treatment (Day 28). The 24 mg/day iloperidone dose was superior to placebo in the PANSS total score. Iloperidone tablets appeared to have similar efficacy to the active control drug which also needed a slow titration to the target dose.
In a longer-term trial, clinically stable adult outpatients (n=303) meeting DSM-IV criteria for schizophrenia who remained stable following 12 weeks of open-label treatment with flexible doses of iloperidone (8 mg/day to 24 mg/day administered as twice daily doses) were randomized to placebo or to continue on their current iloperidone dose (8 mg/day to 24 mg/day administered as twice daily doses) for observation for possible relapse during the double-blind relapse prevention phase. Stabilization during the open-label phase was defined as being on an established dose of iloperidone that was unchanged due to efficacy in the 4 weeks prior to randomization, having CGI-Severity score of ≤4 and PANSS total score ≤70, a score of ≤4 on each of the following individual PANSS items (P1-delusions, P2-conceptual disorganization, P3-hallucinatory behavior, P6-suspiciousness/persecution, P7-hostility, or G8-uncooperativeness), and no hospitalization or increase in level of care to treat exacerbations. Relapse or impending relapse during the double-blind relapse prevention phase was defined as any of the following: hospitalization due to worsening of schizophrenia, increase (worsening) of the PANSS total score ≥30%, CGI-Improvement score ≥6, patient had suicidal, homicidal, or aggressive behavior, or need for any other antipsychotic medication.
Figure 1: Kaplan Meier Estimation of Percent Relapse/Impending Relapse for Iloperidone (Ilo) and Placebo (Pbo)

Based on the interim analysis, an independent data monitoring committee decided the study should be discontinued early due to evidence of efficacy. Based on results from the interim analysis, which were confirmed by the final analysis dataset, patients treated with iloperidone tablets experienced a statistically significant longer time to relapse or impending relapse than patients who received placebo. Figure 1 displays the estimated cumulative proportion of patients with relapse or impending relapse based on the final data set.
16 HOW SUPPLIED/STORAGE AND HANDLING
Iloperidone tablets are available in the following strengths: 1 mg, 2 mg, 4 mg, 6 mg, 8 mg, 10 mg, and 12 mg. The oval (1 mg) white tablets are engraved with "T" on one side and on the other side the tablet strength. The round (2 mg, 4 mg, 6 mg, 8 mg, 10 mg, or 12 mg) white tablets are engraved with "T" on one side and on the other side the tablet strength. Tablets are supplied in the following package configurations:
| Package Configuration | Tablet Strength (mg) | NDC Number |
|---|---|---|
| Bottles of 60 | 1 mg | 51672-4178-4 |
| Bottles of 60 | 2 mg | 51672-4179-4 |
| Bottles of 60 | 4 mg | 51672-4180-4 |
| Bottles of 60 | 6 mg | 51672-4181-4 |
| Bottles of 60 | 8 mg | 51672-4182-4 |
| Bottles of 60 | 10 mg | 51672-4183-4 |
| Bottles of 60 | 12 mg | 51672-4184-4 |
| Titration Pack | 2×1 mg, 2×2 mg, 2×4 mg, 2×6 mg (Total of 8 tablets) | 51672-4213-7 |
17 PATIENT COUNSELING INFORMATION
Physicians are advised to discuss the following issues with patients for whom they prescribe iloperidone tablets:
QT Interval Prolongation
Patients should be advised to consult their physician immediately if they feel faint, lose consciousness or have heart palpitations. Patients should be counseled not to take iloperidone tablets with other drugs that cause QT interval prolongation [see Warnings and Precautions (5.3)]. Patients should be told to inform physicians that they are taking iloperidone tablets before any new drug is taken.
Neuroleptic Malignant Syndrome
Patients and caregivers should be counseled that a potentially fatal symptom complex sometimes referred to as NMS has been reported in association with administration of antipsychotic drugs, including iloperidone tablets. Signs and symptoms of NMS include hyperpyrexia, muscle rigidity, altered mental status, and evidence of autonomic instability (irregular pulse or blood pressure, tachycardia, diaphoresis, and cardiac dysrhythmia) [see Warnings and Precautions (5.4)].
Metabolic Changes
Patients should be aware of the symptoms of hyperglycemia (high blood sugar) and diabetes mellitus. Patients who are diagnosed with diabetes, those with risk factors for diabetes, or those who develop these symptoms during treatment should have their blood glucose monitored at the beginning of and periodically during treatment. Patients should be counseled that weight gain has occurred during treatment with iloperidone tablets. Clinical monitoring of weight is recommended [see Warnings and Precautions (5.6)].
Orthostatic Hypotension
Patients should be advised of the risk of orthostatic hypotension, particularly at the time of initiating treatment, re-initiating treatment, or increasing the dose [see Warnings and Precautions (5.8)].
Interference with Cognitive and Motor Performance
Because iloperidone tablets may have the potential to impair judgment, thinking, or motor skills, patients should be cautioned about operating hazardous machinery, including automobiles, until they are reasonably certain that iloperidone therapy does not affect them adversely [see Warnings and Precautions (5.16)].
Pregnancy
Advise patients that third trimester use of iloperidone tablets may cause extrapyramidal and/or withdrawal symptoms in a neonate. Advise patients to notify their healthcare provider with known or suspected pregnancy [see Use in Specific Populations (8)].
Pregnancy Registry
Advise patients that there is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to iloperidone during pregnancy [see Use in Specific Populations (8)].
Lactation
Advise women not to breastfeed during treatment with iloperidone tablets [see Use in Specific Populations (8.2)].
Concomitant Medication
Patients should be advised to inform their physicians if they are taking, or plan to take, any prescription or over-the-counter drugs, since there is a potential for interactions [see Drug Interactions (7)].
SPL UNCLASSIFIED SECTION
PRINCIPAL DISPLAY PANEL - 1 mg Tablet Bottle Label
PRINCIPAL DISPLAY PANEL - 2 mg Tablet Bottle Label
PRINCIPAL DISPLAY PANEL - 4 mg Tablet Bottle Label
PRINCIPAL DISPLAY PANEL - 6 mg Tablet Bottle Label
PRINCIPAL DISPLAY PANEL - 8 mg Tablet Bottle Label
PRINCIPAL DISPLAY PANEL - 10 mg Tablet Bottle Label
PRINCIPAL DISPLAY PANEL - 12 mg Tablet Bottle Label
PRINCIPAL DISPLAY PANEL - Kit Titration Pack
INGREDIENTS AND APPEARANCE
| ILOPERIDONE
iloperidone tablet |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| ILOPERIDONE
iloperidone tablet |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| ILOPERIDONE
iloperidone tablet |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| ILOPERIDONE
iloperidone tablet |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| ILOPERIDONE
iloperidone tablet |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| ILOPERIDONE
iloperidone tablet |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| ILOPERIDONE
iloperidone tablet |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| ILOPERIDONE
iloperidone kit |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Taro Pharmaceuticals U.S.A., Inc. (145186370) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Taro Pharmaceutical Industries Ltd. | 600072078 | MANUFACTURE(51672-4178, 51672-4179, 51672-4180, 51672-4181, 51672-4182, 51672-4183, 51672-4184, 51672-4213) | |