Search by Drug Name or NDC
NDC 55111-0557-35 Fexofenadine HCl and Pseudoephedrine HCI 180; 240 mg/1; mg/1 Details
Fexofenadine HCl and Pseudoephedrine HCI 180; 240 mg/1; mg/1
Fexofenadine HCl and Pseudoephedrine HCI is a ORAL TABLET, EXTENDED RELEASE in the HUMAN OTC DRUG category. It is labeled and distributed by Dr.Reddy's Laboratories Limited. The primary component is FEXOFENADINE HYDROCHLORIDE; PSEUDOEPHEDRINE HYDROCHLORIDE.
MedlinePlus Drug Summary
The combination of fexofenadine and pseudoephedrine is used in adults and children 12 years of age and older to relieve the allergy symptoms of seasonal allergic rhinitis ('hay fever'), including runny nose; sneezing; congestion (stuffy nose); red, itchy, or watery eyes; or itching of the nose, throat, or roof of the mouth. Fexofenadine is in a class of medications called antihistamines. It works by blocking the effects of histamine, a substance in the body that causes allergy symptoms. Pseudoephedrine is in a class of medications called decongestants. It works by drying up the nasal passages.
Related Packages: 55111-0557-35Last Updated: 11/30/2022
MedLinePlus Full Drug Details: Fexofenadine and Pseudoephedrine
Product Information
| NDC | 55111-0557 |
|---|---|
| Product ID | 55111-557_29216032-68a5-566a-5eaf-ffc9cb597e9a |
| Associated GPIs | 43993002687520 |
| GCN Sequence Number | 058869 |
| GCN Sequence Number Description | fexofenadine/pseudoephedrine TAB ER 24H 180-240MG ORAL |
| HIC3 | Z2O |
| HIC3 Description | 2ND GEN ANTIHISTAMINE AND DECONGESTANT COMBINATION |
| GCN | 24394 |
| HICL Sequence Number | 016846 |
| HICL Sequence Number Description | FEXOFENADINE HCL/PSEUDOEPHEDRINE HCL |
| Brand/Generic | Generic |
| Proprietary Name | Fexofenadine HCl and Pseudoephedrine HCI |
| Proprietary Name Suffix | n/a |
| Non-Proprietary Name | Fexofenadine HCl and Pseudoephedrine HCI |
| Product Type | HUMAN OTC DRUG |
| Dosage Form | TABLET, EXTENDED RELEASE |
| Route | ORAL |
| Active Ingredient Strength | 180; 240 |
| Active Ingredient Units | mg/1; mg/1 |
| Substance Name | FEXOFENADINE HYDROCHLORIDE; PSEUDOEPHEDRINE HYDROCHLORIDE |
| Labeler Name | Dr.Reddy's Laboratories Limited |
| Pharmaceutical Class | Adrenergic alpha-Agonists [MoA], Histamine H1 Receptor Antagonists [MoA], Histamine-1 Receptor Antagonist [EPC], alpha-Adrenergic Agonist [EPC] |
| DEA Schedule | n/a |
| Marketing Category | ANDA |
| Application Number | ANDA079043 |
| Listing Certified Through | 2024-12-31 |
Package
Package Images
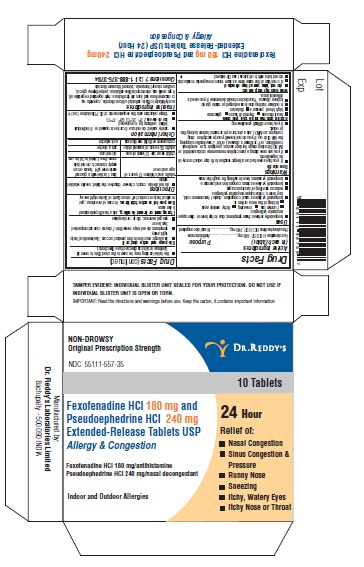
NDC 55111-0557-35 (55111055735)
| NDC Package Code | 55111-557-35 |
|---|---|
| Billing NDC | 55111055735 |
| Package | 2 BLISTER PACK in 1 CARTON (55111-557-35) / 5 TABLET, EXTENDED RELEASE in 1 BLISTER PACK |
| Marketing Start Date | 2011-08-24 |
| NDC Exclude Flag | N |
| Pricing Information | N/A |
Standard Product Labeling (SPL)/Prescribing Information SPL 25cca459-fdd6-3847-2207-dd4f019781b5 Details
Use(s)
- temporarily relieves these symptoms due to hay fever or other upper respiratory allergies:
- runny nose
- sneezing
- itchy, watery eyes
- itching of the nose or throat
- temporarily relieves nasal congestion due to the common cold, hay fever or other upper respiratory allergies
- reduces swelling of nasal passages
- temporarily relieves sinus congestion and pressure
- temporarily restores freer breathing through the nose
Warnings
Do not use
- if you have ever had an allergic reaction to this product or any of its ingredients
- if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson’s disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
- if you have difficulty swallowing
Ask a doctor before use if you have
- heart disease
- thyroid disease
- glaucoma
- high blood pressure
- diabetes
- trouble urinating due to an enlarged prostate gland
- kidney disease. Your doctor should determine if you need a different dose.
When using this product
- do not take more than directed
- do not take at the same time as aluminum or magnesium antacids
- do not take with fruit juices (see Directions)
- the tablet coating may be seen in the stool (this is normal). Continue to take as directed (see Directions).
Directions
- do not divide, crush, chew or dissolve the tablet; swallow tablet whole
| adults and children 12 years of age and over | take 1 tablet with a glass of water every 24 hours on an empty stomach; do not take more than 1 tablet in 24 hours |
| children under 12 years of age | do not use |
| adults 65 years of age and older | ask a doctor |
| consumers with kidney disease | ask a doctor |
Other information
Inactive ingredients
Questions?
INGREDIENTS AND APPEARANCE
| FEXOFENADINE HCL AND PSEUDOEPHEDRINE HCI
fexofenadine hcl and pseudoephedrine hci tablet, extended release |
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| Labeler - Dr.Reddy's Laboratories Limited (650562841) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Dr.Reddy's Laboratories Limted (FTO III) | 918608162 | analysis(55111-557) , manufacture(55111-557) | |