Search by Drug Name or NDC
NDC 55150-0191-83 Ibandronate Sodium 3 mg/3mL Details
Ibandronate Sodium 3 mg/3mL
Ibandronate Sodium is a INTRAVENOUS INJECTION, SOLUTION in the HUMAN PRESCRIPTION DRUG category. It is labeled and distributed by AuroMedics Pharma LLC. The primary component is IBANDRONATE SODIUM.
MedlinePlus Drug Summary
Ibandronate injection is used to treat osteoporosis (a condition in which the bones become thin and weak and break easily) in women who have undergone menopause (''change of life;'' end of menstrual periods). Ibandronate is in a class of medications called bisphosphonates. It works by preventing bone breakdown and increasing bone density (thickness).
Related Packages: 55150-0191-83Last Updated: 11/30/2022
MedLinePlus Full Drug Details: Ibandronate Injection
Product Information
| NDC | 55150-0191 |
|---|---|
| Product ID | 55150-191_1f7e14bc-bafe-4ecb-8679-5d23900f9c05 |
| Associated GPIs | 30042048102030 |
| GCN Sequence Number | 060257 |
| GCN Sequence Number Description | ibandronate sodium SYRINGE 3 MG/3 ML INTRAVEN |
| HIC3 | P4L |
| HIC3 Description | BONE RESORPTION INHIBITORS |
| GCN | 26368 |
| HICL Sequence Number | 017291 |
| HICL Sequence Number Description | IBANDRONATE SODIUM |
| Brand/Generic | Generic |
| Proprietary Name | Ibandronate Sodium |
| Proprietary Name Suffix | n/a |
| Non-Proprietary Name | Ibandronate Sodium |
| Product Type | HUMAN PRESCRIPTION DRUG |
| Dosage Form | INJECTION, SOLUTION |
| Route | INTRAVENOUS |
| Active Ingredient Strength | 3 |
| Active Ingredient Units | mg/3mL |
| Substance Name | IBANDRONATE SODIUM |
| Labeler Name | AuroMedics Pharma LLC |
| Pharmaceutical Class | Bisphosphonate [EPC], Diphosphonates [CS] |
| DEA Schedule | n/a |
| Marketing Category | ANDA |
| Application Number | ANDA205332 |
| Listing Certified Through | 2024-12-31 |
Package
Package Images


NDC 55150-0191-83 (55150019183)
| NDC Package Code | 55150-191-83 |
|---|---|
| Billing NDC | 55150019183 |
| Package | 1 SYRINGE, GLASS in 1 CARTON (55150-191-83) / 3 mL in 1 SYRINGE, GLASS |
| Marketing Start Date | 2015-08-19 |
| NDC Exclude Flag | N |
| Pricing Information | N/A |
Standard Product Labeling (SPL)/Prescribing Information SPL e81f2c5b-bead-48ac-be3e-016cf963d7ed Details
HIGHLIGHTS OF PRESCRIBING INFORMATION
IBANDRONATE SODIUM injection, for intravenous use
Initial U.S. Approval: 2003
INDICATIONS AND USAGE
DOSAGE AND ADMINISTRATION
- 3 mg every 3 months administered intravenously over a period of 15 to 30 seconds (2.2)
- Dosing Instructions:
- Instruct patients to take supplemental calcium and vitamin D if dietary intake is inadequate
DOSAGE FORMS AND STRENGTHS
Ibandronate sodium injection is supplied as a kit containing:
- a 3 mg/3 mL (1 mg/mL) single-dose prefilled syringe.
- a 25-gauge, 3/4 inch needle with wings, needle-stick protection device, and a 9 cm plastic tubing for attachment (3)
WARNINGS AND PRECAUTIONS
- Hypocalcemia can worsen. Correct hypocalcemia prior to use. Adequately supplement patients with calcium and vitamin D (5.1)
- Anaphylaxis, including fatal events, has been reported. (5.2)
- Renal Toxicity may be greater in patients with underlying renal impairment. Do not administer ibandronate sodium injection to patients with severe renal impairment (creatinine clearance less than 30 mL/min). Monitor serum creatinine prior to each dose. (5.3)
- Tissue Damage with Inappropriate Drug Administration can occur. Do not administer ibandronate sodium injection intra-arterially or paravenously. (5.4)
- Osteonecrosis of the jaw (ONJ) has been reported. (5.5)
- Severe Bone, Joint, and/or Muscle Pain may occur, consider discontinuing use if symptoms occur. (5.6)
- Atypical Femur Fractures have been reported. Patients with new thigh or groin pain should be evaluated to rule out a femoral fracture. (5.7)
ADVERSE REACTIONS
The most frequently reported adverse reactions (>5%) are arthralgia, back pain, and abdominal pain. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact AuroMedics Pharma LLC at 1-866-850-2876 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 5/2022
Table Of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 Treatment of Postmenopausal Osteoporosis
1.2 Important Limitations of Use
2 DOSAGE AND ADMINISTRATION
2.1 Important Administration Instructions
2.2 Dosage Information
2.3 Laboratory Testing and Oral Examination Prior to Administration
2.4 Calcium and Vitamin D Supplementation
2.5 Dosing After Missed Dose
2.6 Dosage Modifications in Patients with Renal Impairment
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Hypocalcemia and Mineral Metabolism
5.2 Anaphylactic Reaction
5.3 Renal Impairment
5.4 Tissue Damage Related to Inappropriate Drug Administration
5.5 Osteonecrosis of the Jaw
5.6 Musculoskeletal Pain
5.7 Atypical Subtrochanteric and Diaphyseal Femoral Fractures
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Melphalan/Prednisolone
7.2 Tamoxifen
7.3 Bone Imaging Agents
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
13.2 Animal Pharmacology
14 CLINICAL STUDIES
14.1 Treatment of Postmenopausal Osteoporosis
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage and Handling
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
1 INDICATIONS AND USAGE
1.1 Treatment of Postmenopausal Osteoporosis
Ibandronate sodium injection is indicated for the treatment of osteoporosis in postmenopausal women. In postmenopausal women with osteoporosis, ibandronate sodium injection increases bone mineral density (BMD) and reduces the incidence of vertebral fractures [see Clinical Studies (14)].
1.2 Important Limitations of Use
The safety and effectiveness of ibandronate sodium injection for the treatment of osteoporosis are based on clinical data of one year duration. The optimal duration of use has not been determined. All patients on bisphosphonate therapy should have the need for continued therapy re-evaluated on a periodic basis. Patients at low-risk for fracture should be considered for drug discontinuation after 3 to 5 years of use. Patients who discontinue therapy should have their risk for fracture re-evaluated periodically.
2 DOSAGE AND ADMINISTRATION
2.1 Important Administration Instructions
Ibandronate sodium injection must be administered intravenously only by a health care professional. Care must be taken not to administer intra-arterially or paravenously as this could lead to tissue damage [see Warnings and Precautions (5.4)].
- Appropriate medical support and monitoring measures should be readily available when ibandronate sodium injection is administered. If anaphylactic or other severe hypersensitivity/allergic reactions occur, immediately discontinue the injection and initiate appropriate treatment [see Warnings and Precautions (5.2)].
- Visually inspect the liquid in the prefilled syringe for particulate matter and discoloration before administration. Do not use prefilled syringes with particulate matter or discoloration.
- Administer only with the enclosed needle.
- Discard any unused portion.
- Do not mix with calcium-containing solutions or other intravenously administered drugs.
- Prefilled syringes are single-dose only.
2.2 Dosage Information
The recommended dose of ibandronate sodium injection for the treatment of postmenopausal osteoporosis is 3 mg every 3 months administered intravenously over a period of 15 to 30 seconds. Do not administer more frequently than once every 3 months.
2.3 Laboratory Testing and Oral Examination Prior to Administration
Prior to administration of each dose obtain a serum creatinine [see Warnings and Precautions (5.3)]. Given that bisphosphonates have been associated with osteonecrosis of the jaw (ONJ), perform a routine oral examination prior to administration of ibandronate sodium injection.
2.4 Calcium and Vitamin D Supplementation
Instruct patients to take supplemental calcium and vitamin D if their dietary intake is inadequate [see Warnings and Precautions (5.1)].
2.5 Dosing After Missed Dose
If the dose is missed, administer as soon as it can be re-scheduled. Thereafter, ibandronate sodium injection should be scheduled every 3 months from the date of the last injection.
2.6 Dosage Modifications in Patients with Renal Impairment
Do not administer to patients with severe renal impairment (creatinine clearance less than 30 mL/minute) [see Warnings and Precautions (5.3) and Clinical Pharmacology (12.3)]. No dose adjustment is necessary for patients with mild or moderate renal impairment (creatinine clearance greater than or equal to 30 mL/min) [see Clinical Pharmacology (12.3)].
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
Ibandronate sodium injection is contraindicated in patients with the following conditions:
- Hypocalcemia [see Warnings and Precautions (5.1)]
- Known hypersensitivity to ibandronate sodium injection or to any of its excipients. Cases of anaphylaxis, including fatal events, have been reported. [see Warnings and Precautions (5.2), Adverse Reactions (6.2)]
5 WARNINGS AND PRECAUTIONS
5.1 Hypocalcemia and Mineral Metabolism
Ibandronate sodium injection may cause a decrease in serum calcium values. Treat hypocalcemia, hypovitaminosis D, and other disturbances of bone and mineral metabolism before starting ibandronate sodium injection therapy.
Adequate intake of calcium and vitamin D is important in all patients. It is recommended that patients receive supplemental calcium and vitamin D if dietary intake is inadequate.
5.2 Anaphylactic Reaction
Cases of anaphylaxis, including fatal events, have been reported in patients treated with ibandronate sodium injection.
Appropriate medical support and monitoring measures should be readily available when ibandronate sodium injection is administered. If anaphylactic or other severe hypersensitivity/allergic reactions occur, immediately discontinue the injection and initiate appropriate treatment.
5.3 Renal Impairment
Treatment with intravenous bisphosphonates has been associated with renal toxicity manifested as deterioration in renal function and acute renal failure. Although no cases of acute renal failure were observed in controlled clinical trials in which intravenous ibandronate was administered as a 15- to 30-second bolus, acute renal failure has been reported postmarketing. Do not administer ibandronate sodium injection to patients with severe renal impairment (creatinine clearance less than 30 mL/min).
Obtain serum creatinine prior to each ibandronate sodium injection. After ibandronate sodium injection, assess renal function, as clinically appropriate, in patients with concomitant diseases or taking medications that have the potential for adverse effects on the kidney. Ibandronate sodium injection should be withheld in patients with renal deterioration.
5.4 Tissue Damage Related to Inappropriate Drug Administration
Ibandronate sodium injection must only be administered intravenously. Care must be taken not to administer ibandronate sodium injection intra-arterially or paravenously as this could lead to tissue damage.
Do not administer ibandronate sodium injection by any other route of administration. The safety and efficacy of ibandronate sodium injection following non-intravenous routes of administration have not been established.
5.5 Osteonecrosis of the Jaw
Osteonecrosis of the jaw (ONJ) has been reported in patients treated with bisphosphonates, including ibandronate sodium injection. Most cases have been in cancer patients treated with intravenous bisphosphonates undergoing dental procedures. Some cases have occurred in patients with postmenopausal osteoporosis treated with either oral or intravenous bisphosphonates. A routine oral examination should be performed by the prescriber prior to initiation of bisphosphonate treatment. Consider a dental examination with appropriate preventive dentistry prior to treatment with bisphosphonates in patients with a history of concomitant risk factors (e.g., cancer, chemotherapy, angiogenesis inhibitors, radiotherapy, corticosteroids, poor oral hygiene, pre-existing dental disease or infection, anemia, coagulopathy). Concomitant administration of drugs associated with ONJ may increase the risk of developing ONJ. The risk of ONJ may increase with duration of exposure to bisphosphonates.
While on treatment, patients with concomitant risk factors should avoid invasive dental procedures if possible. For patients who develop ONJ while on bisphosphonate therapy, dental surgery may exacerbate the condition. For patients requiring dental procedures, there are no data available to suggest whether discontinuation of bisphosphonate treatment reduces the risk of ONJ. The clinical judgment of the treating physician should guide the management plan of each patient based on individual benefit/risk assessment [see Adverse Reactions (6.1)].
5.6 Musculoskeletal Pain
Severe and occasionally incapacitating bone, joint, and/or muscle pain has been reported in patients taking ibandronate and other bisphosphonates [see Adverse Reactions (6.2)]. The time to onset of symptoms varied from one day to several months after starting the drug. Most patients had relief of symptoms after stopping the bisphosphonate. A subset of patients had recurrence of symptoms when rechallenged with the same drug or another bisphosphonate. Discontinue ibandronate if severe symptoms develop.
5.7 Atypical Subtrochanteric and Diaphyseal Femoral Fractures
Atypical, low-energy, or low-trauma fractures of the femoral shaft have been reported in bisphosphonate-treated patients. These fractures can occur anywhere in the femoral shaft from just below the lesser trochanter to above the supracondylar flare and are transverse or short oblique in orientation without evidence of comminution. Causality has not been established as these fractures also occur in osteoporotic patients who have not been treated with bisphosphonates.
Atypical femur fractures most commonly occur with minimal or no trauma to the affected area. They may be bilateral and many patients report prodromal pain in the affected area, usually presenting as dull, aching thigh pain, weeks to months before a complete fracture occurs. A number of reports note that patients were also receiving treatment with glucocorticoids (e.g., prednisone) at the time of fracture.
Any patient with a history of bisphosphonate exposure who presents with thigh or groin pain should be suspected of having an atypical fracture and should be evaluated to rule out an incomplete femur fracture. Patients presenting with an atypical fracture should also be assessed for symptoms and signs of fracture in the contralateral limb. Interruption of bisphosphonate therapy should be considered, pending a risk/benefit assessment, on an individual basis.
6 ADVERSE REACTIONS
Adverse reactions that appear in other sections of the labeling include:
- Hypocalcemia and Mineral Metabolism [see Warnings and Precautions (5.1)]
- Anaphylactic Reaction [see Warnings and Precautions (5.2)]
- Renal Impairment [see Warnings and Precautions (5.3)]
- Tissue Damage Related to Inappropriate Drug Administration [see Warnings and Precautions (5.4)]
- Osteonecrosis of the Jaw [see Warnings and Precautions (5.5)]
- Musculoskeletal Pain [see Warnings and Precautions (5.6)]
- Atypical Subtrochanteric and Diaphyseal Femoral Fractures [see Warnings and Precautions (5.7)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Quarterly Intravenous Injection –
In a 1-year, double-blind, multicenter study comparing ibandronate sodium injection administered intravenously as 3 mg every 3 months to ibandronate 2.5 mg daily oral tablet in women with postmenopausal osteoporosis, the overall safety and tolerability profiles of the two dosing regimens were similar. The incidence of serious adverse reactions was 8% in the ibandronate 2.5 mg daily group and 7.5% in the ibandronate sodium injection 3 mg once every 3 months group. The percentage of patients who withdrew from treatment due to adverse reactions was approximately 6.7% in the ibandronate 2.5 mg daily group and 8.5% in the ibandronate sodium injection 3 mg every 3 months group. Table 1 lists the adverse reactions reported in greater than 2% of patients.
| * Combination of abdominal pain and abdominal pain upper † Combination of influenza-like illness and acute phase reaction ‡ Combination of rash, rash pruritic, rash macular, dermatitis, dermatitis allergic, exanthema, erythema, rash papular, rash generalized, dermatitis medicamentosa, rash erythematous |
||
| Body System/Adverse Reaction | Ibandronate
2.5 mg Daily (Oral) % (n = 465) | Ibandronate Sodium Injection
3 mg every 3 months (Intravenous) % (n = 469) |
| Infections and Infestations
|
||
| Influenza | 8 | 5 |
| Nasopharyngitis | 6 | 3 |
| Cystitis | 3 | 2 |
| Gastroenteritis | 3 | 2 |
| Urinary Tract Infection | 3 | 3 |
| Bronchitis | 3 | 2 |
| Upper Respiratory Tract Infection | 3 | 1 |
| Gastrointestinal Disorders
|
||
| Abdominal Pain* | 6 | 5 |
| Dyspepsia | 4 | 4 |
| Nausea | 4 | 2 |
| Constipation | 4 | 3 |
| Diarrhea | 2 | 3 |
| Gastritis | 2 | 2 |
| Musculoskeletal and Connective Tissue Disorders
|
||
| Arthralgia | 9 | 10 |
| Back Pain | 8 | 7 |
| Localized Osteoarthritis | 2 | 2 |
| Pain in Extremity | 2 | 3 |
| Myalgia | 1 | 3 |
| Nervous System Disorders
|
||
| Dizziness | 3 | 2 |
| Headache | 3 | 4 |
| Psychiatric Disorders
|
||
| Insomnia
| 3 | 1 |
| Depression | 2 | 1 |
| General Disorders and Administration Site Conditions
|
||
| Influenza-like Illness†
| 1 | 5 |
| Fatigue | 1 | 3 |
| Skin and Subcutaneous Tissue Disorders
|
||
| Rash‡
| 3 | 2 |
Acute Phase Reaction-like Events
Symptoms consistent with acute phase reaction (APR) have been reported with intravenous bisphosphonate use. The overall incidence of patients with APR-like events was higher in the intravenous treatment group (4% in the ibandronate 2.5 mg daily oral tablet group vs. 10% in the ibandronate sodium injection 3 mg once every 3 months group). These incidence rates are based upon reporting of any of 33 potential APR-like symptoms within 3 days of an intravenous dose and lasting 7 days or less. In most cases, no specific treatment was required and the symptoms subsided within 24 to 48 hours.
Injection Site Reactions
Local reactions at the injection site, such as redness or swelling, were observed at a higher incidence in patients treated with ibandronate sodium injection 3 mg every 3 months (1.7%; 8/469) than in patients treated with placebo injections (0.2%; 1/465). In most cases, the reaction was of mild to moderate severity.
Daily Oral Tablet -
The safety of ibandronate 2.5 mg once daily in the treatment and prevention of postmenopausal osteoporosis was assessed in 3577 patients aged 41 to 82 years. The duration of the trials was 2 to 3 years, with 1134 patients exposed to placebo and 1140 exposed to ibandronate 2.5 mg. Patients with pre-existing gastrointestinal disease and concomitant use of non-steroidal anti-inflammatory drugs, proton pump inhibitors and H2 antagonists were included in these clinical trials. All patients received 500 mg calcium plus 400 international units vitamin D supplementation daily.
The incidence of all-cause mortality was 1% in the placebo group and 1.2% in the ibandronate 2.5 mg daily group. The incidence of serious adverse reactions was 20% in the placebo group and 23% in the ibandronate 2.5 mg daily oral tablet group. The percentage of patients who withdrew from treatment due to adverse reactions was approximately 17% in both the placebo group and the ibandronate 2.5 mg daily oral tablet group. Table 2 lists adverse reactions from the Treatment and Prevention Studies reported in greater than or equal to 2% of patients and in more patients treated with ibandronate 2.5 mg daily oral tablet than patients treated with placebo.
|
Body System | Placebo % (n = 1134) | Ibandronate 2.5 mg daily % (n = 1140) |
| Body as a Whole
|
||
| Back Pain | 12 | 14 |
| Pain in Extremity | 6 | 8 |
| Asthenia | 2 | 4 |
| Allergic Reaction | 2 | 3 |
| Digestive System
|
||
| Dyspepsia | 10 | 12 |
| Diarrhea | 5 | 7 |
| Tooth Disorder | 2 | 4 |
| Vomiting | 2 | 3 |
| Gastritis | 2 | 2 |
| Musculoskeletal System
|
||
| Myalgia | 5 | 6 |
| Joint Disorder | 3 | 4 |
| Arthritis | 3 | 3 |
| Nervous System
|
||
| Headache | 6 | 7 |
| Dizziness | 3 | 4 |
| Vertigo | 3 | 3 |
| Respiratory System
|
||
| Upper Respiratory Infection | 33 | 34 |
| Bronchitis | 7 | 10 |
| Pneumonia | 4 | 6 |
| Pharyngitis | 2 | 3 |
| Urogenital System
|
||
| Urinary Tract Infection | 4 | 6 |
Gastrointestinal Adverse Reactions
The incidence of selected gastrointestinal adverse reactions in the placebo and ibandronate 2.5 mg daily groups were: dyspepsia (10% vs. 12%), diarrhea (5% vs. 7%), and abdominal pain (5% vs. 6%).
Musculoskeletal Adverse Reactions
The incidence of selected musculoskeletal adverse reactions in the placebo and ibandronate 2.5 mg daily groups were: back pain (12% vs. 14%), arthralgia (14% vs. 14%) and myalgia (5% vs. 6%).
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of ibandronate sodium injection. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Hypersensitivity: Allergic reactions including anaphylaxis with fatalities, angioedema, asthma exacerbation, bronchospasm, rash, Stevens-Johnson syndrome, erythema multiforme, and dermatitis bullous [see Contraindications (4), Warnings and Precautions (5.2)].
Hypocalcemia: Hypocalcemia [see Warnings and Precautions (5.1)].
Renal Toxicity: Acute renal failure [see Warnings and Precautions (5.3)].
Osteonecrosis of the Jaw: Osteonecrosis of the jaw and other oro-facial sites, including the external auditory canal [see Warnings and Precautions (5.5)].
Musculoskeletal Pain: Bone, joint, or muscle pain (musculoskeletal pain), described as severe or incapacitating [see Warnings and Precautions (5.6)].
Atypical Femoral Shaft Fracture: Atypical, low-energy, or low-trauma fractures of the femoral shaft [see Warnings and Precautions (5.7)].
Eye Inflammation: Iritis and uveitis. In some cases with other bisphosphonates, these events did not resolve until the bisphosphonate was discontinued.
7 DRUG INTERACTIONS
7.1 Melphalan/Prednisolone
Intravenous ibandronate (6 mg) did not interact with intravenous melphalan (10 mg/m2) or oral prednisolone (60 mg/m2). [See Clinical Pharmacology (12.3)]
7.2 Tamoxifen
There was no interaction between oral 30 mg tamoxifen and intravenous 2 mg ibandronate [See Clinical Pharmacology (12.3)].
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Ibandronate is not indicated for use in women of reproductive potential. There are no data with ibandronate use in pregnant women to inform any drug-associated risks.
In reproductive toxicity studies in the rat, ibandronate caused obstruction of labor, with maternal periparturient mortality, pup loss and reduced pup weight at greater than or equal to 2 times human exposure at the recommended human intravenous dose of 3 mg. Abnormal pup odontogeny was observed at greater than or equal to 18 times human exposure. In rats dosed during pregnancy, kidney developmental toxicity occurred in offspring at greater than or equal to 47 times human exposure. Also, fetal weight and pup growth were reduced at greater than or equal to 5 times human exposure. In reproductive studies in the rabbit, ibandronate caused maternal mortality, reduced maternal body weight gain, decreased litter size due to increased resorption rate, and decreased fetal weight at 19 times the recommended human dose (see Data).
Data
Animal Data
In pregnant rats given intravenous doses producing greater than or equal to 2 times human exposure from Day 17 post-coitum until Day 20 post-partum, ibandronate treatment resulted in dystocia, maternal mortality, and early postnatal pup loss in all dose groups. Reduced body weight at birth was observed at greater than or equal to 4 times the human exposure. Pups exhibited abnormal odontogeny that decreased food consumption and body weight gain at greater than or equal to 18 times human exposure. Periparturient mortality has also been observed with other bisphosphonates and appears to be a class effect related to inhibition of skeletal calcium mobilization resulting in hypocalcemia and dystocia.
Exposure of pregnant rats during the period of organogenesis resulted in an increased fetal incidence of RPU (renal pelvis ureter) syndrome at an intravenous dose producing greater than or equal to 47 times human exposure. In this spontaneous delivery study, dystocia was counteracted by perinatal calcium supplementation. In rat studies with intravenous dosing during gestation, fetal weight and pup growth were reduced at doses producing greater than or equal to 5 times human exposure.
In pregnant rabbits given intravenous doses during the period of organogenesis, maternal mortality, reduced maternal body weight gain, decreased litter size due to increased resorption rate, and decreased fetal weight were observed at 19 times the recommended human intravenous dose.
Exposure multiples for the rat studies were calculated using human exposure at the recommended intravenous dose of 3 mg every 3 months and were based on cumulative area under the curve (AUC) comparison. Exposure multiples for the rabbit study were calculated for the recommended human intravenous dose of 3 mg every 3 months and were based on cumulative dose/[body surface area] comparison. Doses in pregnant animals were 0.05, 0.1, 0.15, 0.3, 0.5 or 1 mg/kg/day in rats, and 0.03, 0.07, or 0.2 mg/kg/day in rabbits.
8.2 Lactation
Risk Summary
Ibandronate is not indicated for use in women of reproductive potential. There is no information on the presence of ibandronate in human milk, the effects of ibandronate on the breastfed infant, or the effects of ibandronate on milk production. Ibandronate is present in rat milk (see Data). The clinical relevance of this data is unclear.
Data
Animal Data
In lactating rats treated with intravenous doses of 0.08 mg/kg, ibandronate was present in breast milk at concentrations of 8.1 to 0.4 ng/mL from 2 to 24 hours after dose administration. Concentrations in milk averaged 1.5 times plasma concentrations.
8.4 Pediatric Use
Safety and effectiveness of ibandronate in pediatric patients have not been established.
8.5 Geriatric Use
Of the patients receiving ibandronate sodium injection 3 mg every 3 months for 1 year, 51% were over 65 years of age. No overall differences in effectiveness or safety were observed between these patients and younger patients, but greater sensitivity in some older individuals cannot be ruled out.
8.6 Renal Impairment
Ibandronate sodium injection should not be administered to patients with severe renal impairment (creatinine clearance less than 30 mL/min) [see Warnings and Precautions (5.3)].
10 OVERDOSAGE
No cases of overdose were reported in premarketing studies with ibandronate sodium injection. Overdosage with intravenous bisphosphonates may result in hypocalcemia, hypophosphatemia, and hypomagnesemia. Clinically relevant reductions in serum levels of calcium, phosphorus, and magnesium should be corrected by intravenous administration of calcium gluconate, potassium or sodium phosphate, and magnesium sulfate, respectively.
Dialysis would not be beneficial unless it is administered within 2 hours following the overdose.
11 DESCRIPTION
Ibandronate sodium is a nitrogen-containing bisphosphonate that inhibits osteoclast-mediated bone resorption. The chemical name for ibandronate sodium is 3-(N-methyl-N-pentyl)amino-1-hydroxypropane-1,1diphosphonic acid, monosodium salt, monohydrate with the molecular formula C9H22NO7P2Na•H2O and a molecular weight of 359.24. Ibandronate sodium is a white to almost white powder. It is freely soluble in water and practically insoluble in organic solvents. Ibandronate sodium has the following structural formula:

Ibandronate sodium injection is intended for intravenous administration only. Ibandronate sodium injection is available as a sterile, clear, colorless, ready-to-use solution free from visible particles in a prefilled syringe that delivers 3.375 mg of ibandronate monosodium salt monohydrate in 3 mL of solution, equivalent to a dose of 3 mg ibandronate free acid. Inactive ingredients include glacial acetic acid (1.53 mg), sodium acetate trihydrate (0.612 mg), sodium chloride (25.8 mg) and water (q.s. to 3 mL). Glacial acetic acid and sodium acetate trihydrate are used to adjust pH.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The action of ibandronate on bone is based on its affinity for hydroxyapatite, which is part of the mineral matrix of bone. Ibandronate inhibits osteoclast activity and reduces bone resorption and turnover. In postmenopausal women, it reduces the elevated rate of bone turnover, leading to, on average, a net gain in bone mass.
12.2 Pharmacodynamics
In studies of postmenopausal women, ibandronate sodium injection at doses of 0.5 mg to 3 mg produced biochemical changes indicative of inhibition of bone resorption, including decreases of biochemical markers of bone collagen degradation (cross-linked C-telopeptide of Type I collagen [CTX]). Changes in markers of bone formation (osteocalcin) were observed later than changes in resorption markers, as expected, due to the coupled nature of bone resorption and formation.
Year 1 results from an efficacy and safety study comparing ibandronate sodium injection 3 mg every 3 months and ibandronate 2.5 mg daily oral tablet demonstrated that both dosing regimens significantly suppressed serum CTX levels at Months 3, 6, and 12. The median pre-dose or trough serum CTX levels in the intent-to-treat population reached a nadir of 57% (ibandronate sodium injection) and 62% (ibandronate 2.5 mg tablets) below baseline values by Month 6, and remained stable at Month 12 of treatment.
12.3 Pharmacokinetics
Distribution
Area under the serum ibandronate concentrations versus time curve increases in a dose proportional manner after administration of 2 mg to 6 mg by intravenous injection.
After administration, ibandronate either rapidly binds to bone or is excreted into urine. In humans, the apparent terminal volume of distribution is at least 90 L, and the amount of dose removed from the circulation into the bone is estimated to be 40% to 50% of the circulating dose. In one study, in vitro protein binding in human serum was approximately 86% over an ibandronate concentration range of 20 to 2,000 ng/mL (approximate range of maximum serum ibandronate concentrations upon intravenous bolus administration).
Metabolism
There is no evidence that ibandronate is metabolized in humans. Ibandronate does not inhibit human P450 1A2, 2A6, 2C9, 2C19, 2D6, 2E1, and 3A4 isozymes in vitro.
Ibandronate does not undergo hepatic metabolism and does not inhibit the hepatic cytochrome P450 system. Ibandronate is eliminated by renal excretion. Based on a rat study, the ibandronate secretory pathway does not appear to include known acidic or basic transport systems involved in the excretion of other drugs.
Elimination
The portion of ibandronate that is not removed from the circulation via bone absorption is eliminated unchanged by the kidney (approximately 50% to 60% of the administered intravenous dose).
The plasma elimination of ibandronate is multiphasic. Its renal clearance and distribution into bone accounts for a rapid and early decline in plasma concentrations, reaching 10% of Cmax within 3 or 8 hours after intravenous or oral administration, respectively. This is followed by a slower clearance phase as ibandronate redistributes back into the blood from bone. The observed apparent terminal half-life for ibandronate is generally dependent on the dose studied and on assay sensitivity. The observed apparent terminal half-life for intravenous 2 and 4 mg ibandronate after 2 hours of infusion ranges from 4.6 to 15.3 hours and 5 to 25.5 hours, respectively.
Following intravenous administration, total clearance of ibandronate is low, with average values in the range 84 to 160 mL/min. Renal clearance (about 60 mL/min in healthy postmenopausal women) accounts for 50% to 60% of total clearance and is related to creatinine clearance. The difference between the apparent total and renal clearances likely reflects bone uptake of the drug.
Pharmacokinetics in Specific Populations
Pediatrics
The pharmacokinetics of ibandronate have not been studied in patients less than 18 years of age.
Gender
The pharmacokinetics of ibandronate are similar in both men and women.
Geriatric
Since ibandronate is not known to be metabolized, the only difference in ibandronate elimination for geriatric patients versus younger patients is expected to relate to progressive age-related changes in renal function [see Use in Specific Populations (8.5)].
Race
Pharmacokinetic differences due to race have not been studied.
Renal Impairment
Renal clearance of ibandronate in patients with various degrees of renal impairment is linearly related to creatinine clearance (CLcr).
Following a single dose of 0.5 mg ibandronate by intravenous administration, patients with creatinine clearance 40 to 70 mL/min had 55% higher exposure (AUC∞) than the exposure observed in patients with creatinine clearance higher than 90 mL/min. Patients with severe renal impairment (creatinine clearance below 30 mL/min) had more than a two-fold increase in exposure compared to the exposure for patients with creatinine clearance equal to or higher than 80 mL/min [see Dosage and Administration (2.6) and Use in Specific Populations (8.6)].
Hepatic Impairment
No studies have been performed to assess the pharmacokinetics of ibandronate in patients with hepatic impairment since ibandronate is not metabolized in the human liver.
Drug Interaction
Melphalan/Prednisolone
A pharmacokinetic interaction study in multiple myeloma patients demonstrated that intravenous melphalan (10 mg/m2) and oral prednisolone (60 mg/m2) did not interact with 6 mg ibandronate upon intravenous coadministration. Ibandronate did not interact with melphalan or prednisolone.
Tamoxifen
A pharmacokinetic interaction study in healthy postmenopausal women demonstrated that there was no interaction between oral 30 mg tamoxifen and intravenous 2 mg ibandronate.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
In a 104-week carcinogenicity study, doses of 3, 7, or 15 mg/kg/day were administered by oral gavage to Wistar rats (systemic exposures in males and females up to 3 and 1 times, respectively, human exposure). There were no significant drug-related tumor findings in male or female rats. In a 78-week carcinogenicity study, doses of 5, 20, or 40 mg/kg/day were administered by oral gavage to NMRI mice (exposures in males and females up to 96 and 14 times, respectively, human exposure). There were no significant drug-related tumor findings in male or female mice. In a 90-week carcinogenicity study, doses of 5, 20, or 80 mg/kg/day were administered in the drinking water to NMRI mice. A dose-related increased incidence of adrenal subcapsular adenoma/carcinoma was observed in female mice, which was statistically significant at 80 mg/kg/day (32 to 51 times human exposure). The relevance of these findings to humans is unknown.
Exposure multiples comparing human and rodent doses were calculated using human exposure at the recommended intravenous dose of 3 mg every 3 months, based on cumulative AUC comparison.
Mutagenesis
There was no evidence for a mutagenic or clastogenic potential of ibandronate in the following assays: in vitro bacterial mutagenesis assay in Salmonella typhimurium and Escherichia coli (Ames test), mammalian cell mutagenesis assay in Chinese hamster V79 cells, and chromosomal aberration test in human peripheral lymphocytes, each with and without metabolic activation. Ibandronate was not genotoxic in the in vivo mouse micronucleus tests for chromosomal damage.
Impairment of Fertility
In female rats treated from 14 days prior to mating through gestation, decreases in fertility, corpora lutea and implantation sites, and increased preimplantation loss were observed at an intravenous dose of 1.2 mg/kg/day (117 times human exposure). In male rats treated for 28 days prior to mating, a decrease in sperm production and altered sperm morphology were observed at intravenous doses greater than or equal to 0.3 mg/kg/day (greater than or equal to 40 times human exposure).
Exposure multiples comparing human and rat doses were calculated using human exposure at the recommended intravenous dose of 3 mg every 3 months, based on cumulative AUC comparison.
13.2 Animal Pharmacology
Animal studies have shown that ibandronate is an inhibitor of osteoclast-mediated bone resorption. In the Schenk assay in growing rats, ibandronate inhibited bone resorption and increased bone volume, based on histologic examination of the tibial metaphyses. There was no evidence of impaired mineralization at the highest dose of 5 mg/kg/day (subcutaneously), which is 1000 times the lowest antiresorptive dose of 0.005 mg/kg/day in this model, and 5000 times the optimal antiresorptive dose of 0.001 mg/kg/day in the aged ovariectomized rat. This indicates that ibandronate sodium injection administered at a therapeutic dose is unlikely to induce osteomalacia.
Long-term daily or intermittent administration of ibandronate to ovariectomized rats or monkeys was associated with suppression of bone turnover and increases in bone mass. Vertebral BMD, trabecular density, and biomechanical strength were increased dose-dependently in rats and monkeys, at doses up to 8 to 4 times the human intravenous dose of 3 mg every 3 months, based on cumulative dose normalized for body surface area (mg/m2) and area under the curve (AUC) comparison, respectively. Ibandronate maintained the positive correlation between bone mass and strength at the ulna and femoral neck. New bone formed in the presence of ibandronate had normal histologic structure and did not show mineralization defects.
14 CLINICAL STUDIES
14.1 Treatment of Postmenopausal Osteoporosis
Quarterly Intravenous Injection
The effectiveness and safety of ibandronate sodium injection 3 mg once every 3 months were demonstrated in a randomized, double-blind, multinational, noninferiority study in 1358 women with postmenopausal osteoporosis (L2-L4 lumbar spine BMD, T-score below -2.5 SD at baseline). The control group received ibandronate 2.5 mg daily oral tablets. The primary efficacy parameter was the relative change from baseline to 1 year of treatment in lumbar spine BMD, which was compared between the intravenous injection and the daily oral treatment groups. All patients received 400 international units vitamin D and 500 mg calcium supplementation per day.
Effect on BMD
In the intent-to-treat (ITT) efficacy analysis, the least-squares mean increase at 1 year in lumbar spine BMD in patients (n = 429) treated with ibandronate sodium injection 3 mg once every 3 months (4.5%) was statistically superior to that in patients (n = 434) treated with daily oral tablets (3.5%). The mean difference between groups was 1.1% (95% confidence interval: 0.5%, 1.6%; p<0.001; see Figure 1). The mean increase from baseline in total hip BMD at 1 year was 2.1% in the ibandronate sodium injection 3 mg once every 3 months group and 1.5% in the ibandronate 2.5 mg daily oral tablet group. Consistently higher BMD increases at the femoral neck and trochanter were also observed following ibandronate sodium injection 3 mg once every 3 months compared to ibandronate 2.5 mg daily oral tablet.
Figure 1 Mean Percent Change (95% Confidence Interval) from Baseline in Lumbar Spine BMD at One Year in Patients Treated with Ibandronate 2.5 mg Daily Oral Tablet or Ibandronate Sodium Injection 3 mg Once Every 3 Months

Bone Histology
The histological analysis of bone biopsies after 22 months of treatment with 3 mg intravenous ibandronate every 3 months (n = 30) or 23 months of treatment with 2 mg intravenous ibandronate every 2 months (n = 27) in women with postmenopausal osteoporosis showed bone of normal quality and no indication of a mineralization defect.
Daily Oral Tablets
The effectiveness and safety of ibandronate daily oral tablets were demonstrated in a randomized, double-blind, placebo-controlled, multinational study (Treatment Study) of 2946 women aged 55 to 80 years, who were on average 21 years postmenopause, who had a lumbar spine BMD 2 to 5 SD below the premenopausal mean (T-score) in at least one vertebra [L1-L4], and who had one to four prevalent vertebral fractures. Ibandronate was evaluated at oral doses of 2.5 mg daily and 20 mg intermittently. The main outcome measure was the occurrence of new radiographically diagnosed, vertebral fractures after 3 years of treatment. The diagnosis of an incident vertebral fracture was based on both qualitative diagnosis by the radiologist and quantitative morphometric criterion. The morphometric criterion required the dual occurrence of two events: a relative height ratio or relative height reduction in a vertebral body of at least 20%, together with at least a 4 mm absolute decrease in height. All women received 400 international units vitamin D and 500 mg calcium supplementation per day.
Effect on Vertebral Fracture
Ibandronate 2.5 mg daily oral tablet significantly reduced the incidence of new vertebral fractures compared to placebo. Over the course of the 3-year study, the risk for new vertebral fracture was 9.6% in the placebo-treated women and 4.7% in the women treated with ibandronate 2.5 mg daily oral tablet (p<0.001) (see Table 3).
| * The endpoint value is the value at the study’s last time point, 3 years, for all patients who had a fracture identified at that time; otherwise, the last postbaseline value prior to the study’s last time point is used. ** p = 0.0003 vs. placebo ***“Worsening vertebral fracture” defined as a new fracture in a vertebral body with a prevalent fracture |
||||
| | Proportion of Patients with Fracture (%)
|
|||
| | Placebo
n = 975 | Ibandronate
2.5 mg Daily n = 977 | Absolute Risk Reduction
(%) 95% CI | Relative Risk Reduction
(%) 95% CI |
| New Vertebral Fracture 0 to 3 Year | 9.6 | 4.7 | 4.9 (2.3, 7.4) | 52** (29, 68) |
| New and Worsening Vertebral Fracture*** 0 to 3 Year | 10.4 | 5.1 | 5.3 (2.6, 7.9) | 52 (30, 67) |
| Clinical (Symptomatic) Vertebral Fracture 0 to 3 Year | 5.3 | 2.8 | 2.5 (0.6, 4.5) | 49 (14, 69) |
Effect on Nonvertebral Fractures
Ibandronate 2.5 mg daily did not reduce the incidence of nonvertebral fractures (secondary efficacy measure). There was a similar number of nonvertebral osteoporotic fractures at 3 years reported in women treated with ibandronate 2.5 mg daily oral tablet [9.1%, (95% CI: 7.1%, 11.1%)] and placebo [8.2%, (95% CI: 6.3%, 10.2%)]. The two treatment groups were also similar with regard to the number of fractures reported at the individual non-vertebral sites: pelvis, femur, wrist, forearm, rib, and hip.
Effect on BMD
Ibandronate 2.5 mg daily oral tablet significantly increased BMD at the lumbar spine and hip relative to treatment with placebo. In the 3-year osteoporosis treatment study, ibandronate 2.5 mg daily oral tablet produced increases in lumbar spine BMD that were progressive over 3 years of treatment and were statistically significant relative to placebo at 6 months and at all later time points. Lumbar spine BMD increased by 6.4% after 3 years of treatment with ibandronate 2.5 mg daily oral tablet compared with 1.4% in the placebo group (p<0.0001). Table 4 displays the significant increases in BMD seen at the lumbar spine, total hip, femoral neck, and trochanter compared to placebo.
| * The endpoint value is the value at the study’s last time point, 3 years, for all patients who had BMD measured at that time; otherwise the last postbaseline value prior to the study’s last time point is used. |
||
| | Placebo
| Ibandronate
2.5 mg |
| Lumbar Spine | 1.4 (n = 693) | 6.4 (n = 712) |
| Total Hip | -0.7 (n = 638) | 3.1 (n = 654) |
| Femoral Neck | -0.7 (n = 683) | 2.6 (n = 699) |
| Trochanter | 0.2 (n = 683) | 5.3 (n = 699) |
Bone Histology
The effects of ibandronate 2.5 mg daily oral tablet on bone histology were evaluated in iliac crest biopsies from 16 women after 22 months of treatment and 20 women after 34 months of treatment. The histological analysis of bone biopsies showed bone of normal quality and no indication of osteomalacia or a mineralization defect.
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
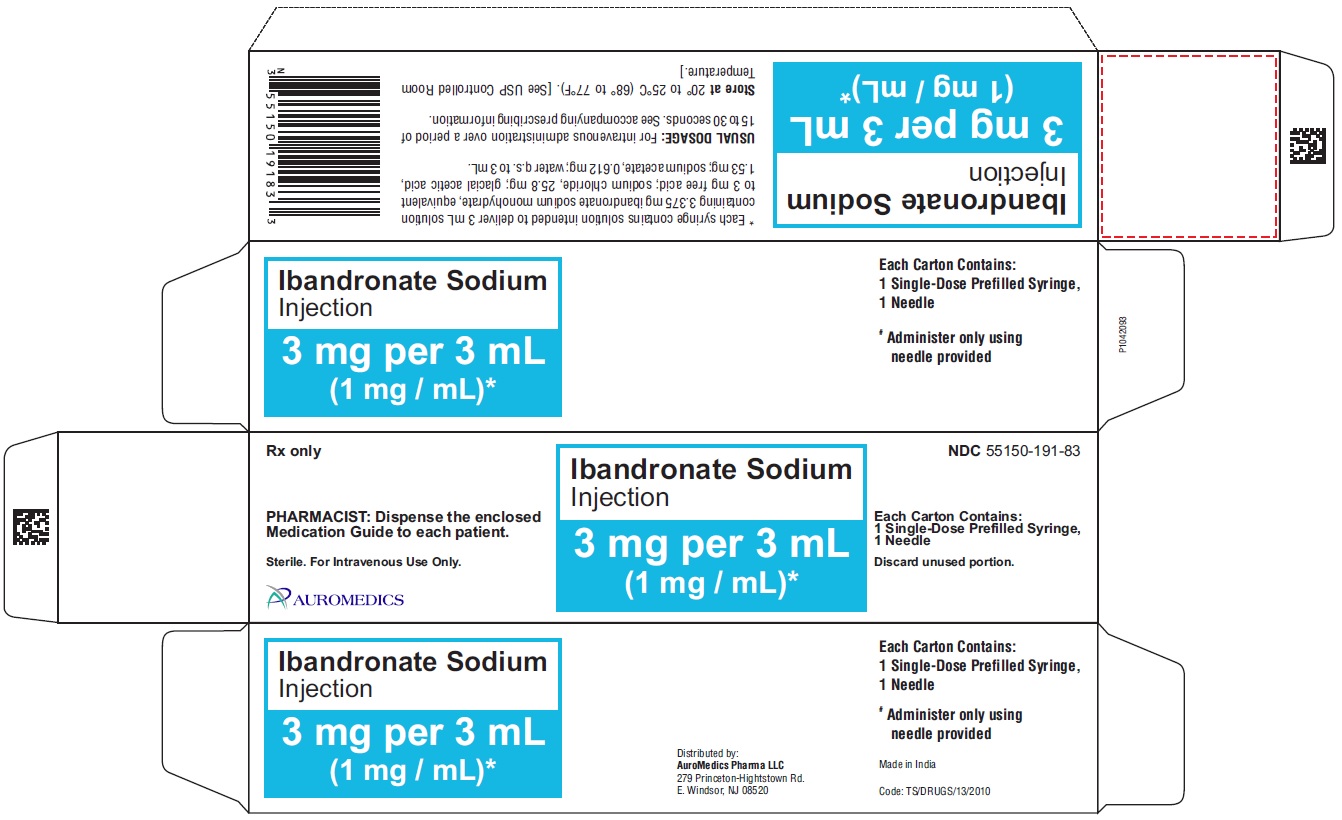
Ibandronate sodium injection is supplied as a kit containing:
- a 3 mg/3 mL (1mg/mL) single-dose prefilled syringe.
- a 25-gauge, 3/4 inch needle with wings, needle-stick protection device, and a 9 cm plastic tubing for attachment.
3 mg per 3 mL (1 mg/mL):
3 mL Single-Dose Prefilled Syringes,
Packaged in a carton with 1 needle NDC 55150-191-83
17 PATIENT COUNSELING INFORMATION
“See FDA-approved patient labeling (Medication Guide)”
Inform patients that ibandronate sodium injection must be administered intravenously by a health care professional.
Patients should be instructed to read the Medication Guide carefully before ibandronate is administered and to re-read it each time the prescription is renewed because it contains important information the patient should know about ibandronate.
Inform patients that ibandronate sodium injection is administered once every 3 months. If the dose is missed, the injection should be administered as soon as it can be rescheduled. Thereafter, injections should be scheduled every 3 months from the date of the last injection. Do not administer ibandronate sodium injection more frequently than once every 3 months.
Inform patients that they should take supplemental calcium and vitamin D if their dietary intake is inadequate [see Warnings and Precautions (5.1)].
Inform patients ibandronate sodium injection should not be administered to patients with creatinine clearance less than 30 mL/min. A serum creatinine should be measured prior to each dose [see Warnings and Precautions (5.3)].
Inform patients that the most common side effects of ibandronate include arthralgia, back pain, hypertension, and abdominal pain. Flu-like symptoms (acute phase reaction) may occur within 3 days following infusion, and usually subside within 24 to 48 hours without specific therapy.
Inform patients that there have been reports of persistent pain and/or a non-healing sore of the mouth or jaw, primarily in patients treated with bisphosphonates for other illnesses. If they experience these symptoms, they should inform their physician or dentist.
Inform patients that severe bone, joint, and/or muscle pain have been reported in patients taking bisphosphonates, including ibandronate. Patients should report severe symptoms if they develop.
Inform patients that atypical femur fractures in patients on bisphosphonate therapy have been reported. Patients should report new thigh or groin pain and undergo evaluation to rule out a femoral fracture.
Distributed by:
AuroMedics Pharma LLC
279 Princeton-Hightstown Rd.
E. Windsor, NJ 08520
Manufactured by:
Eugia Pharma Specialities Limited
Hyderabad - 500032
India
MEDICATION GUIDE
IBANDRONATE SODIUM
(eye ban′ droe nate soe′ dee um)
INJECTION
for intravenous use
Read the Medication Guide that comes with ibandronate sodium injection before you start taking it and each time you get a refill. There may be new information. This Medication Guide does not take the place of talking with your doctor about your medical condition or treatment. Talk to your doctor if you have any questions about ibandronate sodium injection.
What is the most important information I should know about ibandronate sodium injection?
Ibandronate sodium injection is given in your vein (intravenously) and only given by a healthcare provider. Do not give ibandronate sodium injection to yourself.
Ibandronate sodium injection may cause serious side effects including:
- Low calcium levels in your blood (hypocalcemia)
- Severe allergic reaction (anaphylactic reaction)
- Severe kidney problems
- Severe jaw bone problems (osteonecrosis)
- Bone, joint or muscle pain
- Unusual thigh bone fractures
1. Low calcium levels in your blood (hypocalcemia).
Ibandronate sodium injection may lower the calcium levels in your blood. If you have low blood calcium before you start taking ibandronate sodium injection, it may get worse during treatment. Your low blood calcium must be treated before you receive ibandronate sodium injection. Most people with low blood calcium levels do not have symptoms, but some people may have symptoms. Call your doctor right away if you have symptoms of low blood calcium such as:
- Spasms, twitches, or cramps in your muscles
- Numbness or tingling in your fingers, toes, or around your mouth
Your doctor may prescribe calcium and vitamin D to help prevent low calcium levels in your blood, while you receive ibandronate sodium injection. Take calcium and vitamin D as your doctor tells you to.
2. Severe allergic reactions.
Some people who received ibandronate sodium injection had severe allergic reactions (anaphylactic reactions) that led to death. Get medical help right away if you have any of the symptoms of a serious allergic reaction such as:
- Swelling of your face, lips, mouth or tongue
- Trouble breathing
- Wheezing
- Severe itching
- Skin rash, redness or swelling
- Dizziness or fainting
- Fast heartbeat or pounding in your chest
- Sweating
3. Severe kidney problems.
Severe kidney problems, including kidney failure, may happen when you receive ibandronate sodium injection. Your doctor should do blood tests to check your kidneys before you receive each dose of ibandronate sodium injection.
4. Severe jaw bone problems (osteonecrosis).
Severe jaw bone problems may happen when you receive ibandronate sodium injection. Your doctor may examine your mouth before you start ibandronate sodium injection. Your doctor may tell you to see your dentist before you start ibandronate sodium injection. It is important for you to practice good mouth care during treatment with ibandronate sodium injection.
5. Bone, joint, or muscle pain.
Some people who receive ibandronate sodium injection develop severe bone, joint, or muscle pain.
6. Unusual thigh bone fractures.
Some people have developed unusual fractures in their thigh bone. Symptoms of a fracture may include new or unusual pain in your hip, groin, or thigh.
Call your doctor right away if you have any of these side effects.
What is ibandronate sodium injection?
Ibandronate sodium injection is a prescription medicine used to treat osteoporosis in women after menopause. Ibandronate sodium injection helps increase bone mass and helps reduce the chance of having a spinal fracture (break).
It is not known how long ibandronate sodium injection works for the treatment of osteoporosis. You should see your doctor regularly to determine if ibandronate sodium injection is still right for you.
It is not known if ibandronate sodium injection is safe and effective in children.
Who should not receive ibandronate sodium injection?
Do not receive ibandronate sodium injection if you:
- Have low levels of calcium in your blood
- Are allergic to ibandronate sodium or any of the ingredients in ibandronate sodium injection. See the end of this leaflet for a complete list of ingredients in ibandronate sodium injection.
What should I tell my healthcare provider before receiving ibandronate sodium injection?
Before you receive ibandronate sodium injection, tell your doctor if you:
- Have low blood calcium
- Plan to have dental surgery or teeth removed
- Have kidney problems or other problems that may affect your kidneys
- Have been told you have trouble absorbing minerals in your stomach or intestines (malabsorption syndrome)
- Are pregnant or plan to become pregnant. It is not known if ibandronate sodium injection can harm your unborn baby.
- Are breast-feeding or plan to breast-feed. It is not known if ibandronate passes into your milk and may harm your baby.
Tell your doctor and dentist about all the medicines you take, including prescription and non-prescription medicines, vitamins and herbal supplements.
Know the medicines you take. Keep a list of them and show it to your healthcare provider and pharmacist each time you get a new medicine.
How should I receive ibandronate sodium injection?
- Ibandronate sodium injection is given 1 time every 3 months by a healthcare provider.
- If you miss a dose of ibandronate sodium injection, call your doctor or healthcare provider to schedule your next dose.
What are the possible side effects of ibandronate sodium injection?
Ibandronate sodium injection may cause serious side effects.
- See “What is the most important information I should know about ibandronate sodium injection?”
The most common side effects of ibandronate sodium injection include:
- Pain in your bones, joints or muscles
- Back pain
- Abdominal pain
- Flu-like symptoms may happen within 3 days after you receive ibandronate sodium injection. Symptoms include:
- fever
- chills
- bone, joint, or muscle pain
- fatigue
If you have flu-like symptoms, they should get better within 24 to 48 hours.
Some people have pain or a sore that will not heal in their mouth or jaw while they receive ibandronate sodium injection. Tell your doctor or dentist if you have mouth or jaw problems.
Tell your doctor if you have any side effect that bothers you or that does not go away.
These are not all the possible side effects of ibandronate sodium injection. For more information, ask your doctor or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. You may also report side effects to AuroMedics Pharma LLC at 1-866-850-2876.
How should I store ibandronate sodium injection if I need to pick it up from a pharmacy?
- Store ibandronate sodium injection at room temperature between 68°F and 77°F (20°C and 25°C).
Keep ibandronate sodium injection and all medicines out of the reach of children.
General information about the safe and effective use of ibandronate sodium injection.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use ibandronate sodium injection for a condition for which it was not prescribed. Do not give ibandronate sodium injection to other people, even if they have the same symptoms you have. It may harm them.
This Medication Guide summarizes the most important information about ibandronate sodium injection. If you would like more information, talk with your doctor. You can ask your doctor or pharmacist for information about ibandronate sodium injection that is written for health professionals.
What are the ingredients in ibandronate sodium injection?
Active ingredient: ibandronate sodium
Inactive ingredients: glacial acetic acid, sodium acetate trihydrate, sodium chloride, and water
This Medication Guide has been approved by the U.S. Food and Drug Administration.
Distributed by:
AuroMedics Pharma LLC
279 Princeton-Hightstown Rd.
E. Windsor, NJ 08520
Manufactured by:
Eugia Pharma Specialities Limited
Hyderabad - 500032
India
Revised: March 2022
TERUMO® Surflo® Safety Winged Infusion Set —
Instructions for Use: IV Administration
Aseptic technique, proper skin preparation and continued protection of the site are essential. Observe Universal Precautions on all patients.
Caution: Keep hands behind the needle at all times during use and disposal.
Instructions for Device Assembly
- Open the package.
- Remove the rubber cap from the tip of the syringe containing the ibandronate sodium injection and the protective SV cap from hub at the end of the tubing opposite the butterfly needle.
- Insert the tip of the syringe into the hub and twist as firm pressure is applied to assure a tight connection.
- Proceed with priming and confirm that administration fluid comes from the needle.
Venipuncture and Administration
- Flip the safety shield back away from the needle towards the tubing. Grasp wings securely.
- Remove the needle protector. Caution: Care should be taken not to touch the needle.
- Perform venipuncture and confirm proper positioning of the needle in the vein.
- Carefully allow wings to return to starting position and conform to the shape of the skin.
- Further secure the position of the winged infusion set per facility protocol.
After Use
- Remove tape, if present, from wings.
- Flip the safety shield forward toward the needle. Grasp a wing and the safety shield between your thumb and index finger. Completely remove the needle from the puncture site and apply digital pressure to the site using a sterile gauze pad held in the opposite hand (Fig. 1).
- With the wing and shield between your thumb and index finger pinch together (or press the safety shield against a hard surface such as a bedside table) until an audible click is heard (Fig. 2).
- Visually confirm activation of the safety feature (Fig. 3).
- Dispose of used needles and materials following the policies and procedures of your facility, as well as federal and local regulations for “Sharps Disposal.”

®Registered Trademark. Surflo is a registered trademark of TERUMO CORPORATION.
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 3 mg per 3 mL (1 mg / mL) - Prefilled Syringe Label
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 3 mg per 3 mL (1 mg / mL) - Prefilled Syringe Blister Label
Rx only NDC 55150-191-83
Ibandronate Sodium
Injection
3 mg per 3 mL
(1 mg / mL)*
PHARMACIST: Dispense the enclosed Medication Guide to each
patient.
Sterile. For Intravenous Use Only. Single-Dose Prefilled Syringe,
Discard unused portion. Ibandronate Sodium Injection
3 mg per 3 mL (1 mg / mL)
AUROMEDICS

PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 3 mg per 3 mL (1 mg / mL) - Prefilled Syringe-Carton
INGREDIENTS AND APPEARANCE
| IBANDRONATE SODIUM
ibandronate sodium injection, solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - AuroMedics Pharma LLC (968961354) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Aurobindo Pharma Limited | 918917626 | ANALYSIS(55150-191) , API MANUFACTURE(55150-191) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Eugia Pharma Specialities Limited | 650498244 | ANALYSIS(55150-191) , MANUFACTURE(55150-191) | |