Search by Drug Name or NDC
NDC 62332-0545-05 Timolol Maleate Ophthalmic Gel Forming Solution, 0.25% 2.5 mg/mL Details
Timolol Maleate Ophthalmic Gel Forming Solution, 0.25% 2.5 mg/mL
Timolol Maleate Ophthalmic Gel Forming Solution, 0.25% is a OPHTHALMIC SOLUTION/ DROPS in the HUMAN PRESCRIPTION DRUG category. It is labeled and distributed by Alembic Pharmaceuticals Inc.. The primary component is TIMOLOL MALEATE.
MedlinePlus Drug Summary
Ophthalmic timolol is used to treat glaucoma, a condition in which increased pressure in the eye can lead to gradual loss of vision. Timolol is in a class of medications called beta-blockers. It works by decreasing the pressure in the eye.
Related Packages: 62332-0545-05Last Updated: 12/01/2022
MedLinePlus Full Drug Details: Timolol Ophthalmic
Product Information
| NDC | 62332-0545 |
|---|---|
| Product ID | 62332-545_dcd645b8-b1f0-46bc-b20e-83e5db80d01e |
| Associated GPIs | 86250030107620 |
| GCN Sequence Number | 021400 |
| GCN Sequence Number Description | timolol maleate SOL-GEL 0.25 % OPHTHALMIC |
| HIC3 | Q6G |
| HIC3 Description | MIOTICS AND OTHER INTRAOCULAR PRESSURE REDUCERS |
| GCN | 32822 |
| HICL Sequence Number | 002105 |
| HICL Sequence Number Description | TIMOLOL MALEATE |
| Brand/Generic | Generic |
| Proprietary Name | Timolol Maleate Ophthalmic Gel Forming Solution, 0.25% |
| Proprietary Name Suffix | n/a |
| Non-Proprietary Name | Timolol Maleate Ophthalmic Gel Forming Solution, 0.25% |
| Product Type | HUMAN PRESCRIPTION DRUG |
| Dosage Form | SOLUTION/ DROPS |
| Route | OPHTHALMIC |
| Active Ingredient Strength | 2.5 |
| Active Ingredient Units | mg/mL |
| Substance Name | TIMOLOL MALEATE |
| Labeler Name | Alembic Pharmaceuticals Inc. |
| Pharmaceutical Class | Adrenergic beta-Antagonists [MoA], beta-Adrenergic Blocker [EPC] |
| DEA Schedule | n/a |
| Marketing Category | ANDA |
| Application Number | ANDA212942 |
| Listing Certified Through | 2024-12-31 |
Package
Package Images




NDC 62332-0545-05 (62332054505)
| NDC Package Code | 62332-545-05 |
|---|---|
| Billing NDC | 62332054505 |
| Package | 1 BOTTLE in 1 CARTON (62332-545-05) / 5 mL in 1 BOTTLE |
| Marketing Start Date | 2020-11-22 |
| NDC Exclude Flag | N |
| Pricing Information | N/A |
Standard Product Labeling (SPL)/Prescribing Information SPL b909ecf0-c90a-4719-a144-9e74f457cb87 Details
DESCRIPTION
Timolol maleate ophthalmic gel forming solution is a non-selective beta-adrenergic receptor blocking agent. Its chemical name is (-)-1-(tert-butylamino)-3 [(4-morpholino-1,2,5-thiadiazol-3-yl)oxy]-2-propanol maleate (1:1) (salt). Timolol maleate possesses an asymmetric carbon atom in its structure and is provided as the levo-isomer. The optical rotation of timolol maleate is:
25°
[α] in 1.0N HCl (C = 5%) = -12.2° (-11.7° to -12.5°).
405 nm
Its molecular formula is C13H24N4O3S•C4H4O4 and its structural formula is:

Timolol maleate has a molecular weight of 432.50. It is a white, odorless, crystalline powder which is soluble in water, methanol, and alcohol.
Timolol maleate ophthalmic gel forming solution is supplied as a sterile, isotonic, buffered, aqueous solution of timolol maleate in two dosage strengths. The pH of the solution is approximately 7.0, and the osmolarity is 260-330 mOsm. Each mL of Timolol maleate ophthalmic gel forming solution 0.25% contains 2.5 mg of timolol (3.4 mg of timolol maleate). Each mL of Timolol maleate ophthalmic gel forming solution 0.5% contains 5 mg of timolol (6.8 mg of timolol maleate). Inactive ingredients: gellan gum, tromethamine, mannitol, and water for injection. Preservative: benzododecinium bromide 0.012%.
The gel forming solution contains a purified anionic heteropolysaccharide derived from gellan gum. An aqueous solution of gellan gum, in the presence of a cation, has the ability to gel. Upon contact with the precorneal tear film, Timolol maleate ophthalmic gel forming solution forms a gel that is subsequently removed by the flow of tears.
CLINICAL PHARMACOLOGY
Mechanism of Action
Timolol maleate is a beta1 and beta2 (non-selective) adrenergic receptor blocking agent that does not have significant intrinsic sympathomimetic, direct myocardial depressant, or local anesthetic (membrane-stabilizing) activity.
Timolol maleate ophthalmic gel forming solution, when applied topically on the eye, has the action of reducing elevated, as well as normal intraocular pressure, whether or not accompanied by glaucoma. Elevated intraocular pressure is a major risk factor in the pathogenesis of glaucomatous visual field loss and optic nerve damage.
The precise mechanism of the ocular hypotensive action of Timolol maleate ophthalmic gel forming solution is not clearly established at this time. Tonography and fluorophotometry studies of TIMOPTIC® (timolol maleate ophthalmic solution) in man suggest that its predominant action may be related to reduced aqueous formation. However, in some studies, a slight increase in outflow facility was also observed.
Beta-adrenergic receptor blockade reduces cardiac output in both healthy subjects and patients with heart disease. In patients with severe impairment of myocardial function, beta-adrenergic receptor blockade may inhibit the stimulatory effect of the sympathetic nervous system necessary to maintain adequate cardiac function.
Beta-adrenergic receptor blockade in the bronchi and bronchioles results in increased airway resistance from unopposed parasympathetic activity. Such an effect in patients with asthma or other bronchospastic conditions is potentially dangerous.
Pharmacokinetics
In a study of plasma drug concentration in six subjects, the systemic exposure to timolol was determined following once daily administration of Timolol maleate ophthalmic gel forming solution 0.5% in the morning. The mean peak plasma concentration following this morning dose was 0.28 ng/mL.
Clinical Studies
In controlled, double-masked, multicenter clinical studies, comparing Timolol maleate ophthalmic gel forming solution 0.25% to TIMOPTIC 0.25% and Timolol maleate ophthalmic gel forming solution 0.5% to TIMOPTIC 0.5%, Timolol maleate ophthalmic gel forming solution administered once a day was shown to be equally effective in lowering intraocular pressure as the equivalent concentration of TIMOPTIC administered twice a day. The effect of timolol in lowering intraocular pressure was evident for 24 hours with a single dose of Timolol maleate ophthalmic gel forming solution. Repeated observations over a period of six months indicate that the intraocular pressure-lowering effect of Timolol maleate ophthalmic gel forming solution was consistent. The results from the largest U.S. and international clinical trials comparing Timolol maleate ophthalmic gel forming solution 0.5% to TIMOPTIC 0.5% are shown in Figure 1.
Figure 1
Mean IOP and Std Deviation
(mm Hg) by Treatment Group


Timolol maleate ophthalmic gel forming solution administered once daily had a safety profile similar to that of an equivalent concentration of TIMOPTIC administered twice daily. Due to the physical characteristics of the formulation, there was a higher incidence of transient blurred vision in patients administered Timolol maleate ophthalmic gel forming solution. A slight reduction in resting heart rate was observed in some patients receiving Timolol maleate ophthalmic gel forming solution 0.5% (mean reduction 24 hours post-dose 0.8 beats/minute, mean reduction 2 hours post-dose 3.8 beats/minute) [see ADVERSE REACTIONS].
Timolol maleate ophthalmic gel forming solution has not been studied in patients wearing contact lenses.
INDICATIONS AND USAGE
CONTRAINDICATIONS
Timolol maleate ophthalmic gel forming solution is contraindicated in patients with (1) bronchial asthma; (2) a history of bronchial asthma; (3) severe chronic obstructive pulmonary disease [see WARNINGS, Obstructive Pulmonary Disease]; (4) sinus bradycardia; (5) second or third degree atrioventricular block; (6) overt cardiac failure [see WARNINGS, Cardiac Failure]; (7) cardiogenic shock; or (8) hypersensitivity to any component of this product.
WARNINGS
As with many topically applied ophthalmic drugs, this drug is absorbed systemically. The same adverse reactions found with systemic administration of beta adrenergic blocking agents may occur with topical ophthalmic administration. For example, severe respiratory reactions and cardiac reactions, including death due to bronchospasm in patients with asthma, and rarely death in association with cardiac failure, have been reported following systemic or ophthalmic administration of timolol maleate [see CONTRAINDICATIONS].
Cardiac Failure
Sympathetic stimulation may be essential for support of the circulation in individuals with diminished myocardial contractility, and its inhibition by beta-adrenergic receptor blockade may precipitate more severe failure.
In Patients Without a History of Cardiac Failure continued depression of the myocardium with beta-blocking agents over a period of time can, in some cases, lead to cardiac failure. At the first sign or symptom of cardiac failure, Timolol maleate ophthalmic gel forming solution should be discontinued.
Obstructive Pulmonary Disease
Patients with chronic obstructive pulmonary disease (e.g., chronic bronchitis, emphysema) of mild or moderate severity, bronchospastic disease, or a history of bronchospastic disease (other than bronchial asthma or a history of bronchial asthma, in which Timolol maleate ophthalmic gel forming solution is contraindicated [see CONTRAINDICATIONS] should, in general, not receive beta-blockers, including Timolol maleate ophthalmic gel forming solution.
Major Surgery
The necessity or desirability of withdrawal of beta-adrenergic blocking agents prior to major surgery is controversial. Beta-adrenergic receptor blockade impairs the ability of the heart to respond to beta-adrenergically mediated reflex stimuli. This may augment the risk of general anesthesia in surgical procedures. Some patients receiving beta adrenergic receptor blocking agents have experienced protracted, severe hypotension during anesthesia. Difficulty in restarting and maintaining the heartbeat has also been reported. For these reasons, in patients undergoing elective surgery, some authorities recommend gradual withdrawal of beta-adrenergic receptor blocking agents.
If necessary during surgery, the effects of beta-adrenergic blocking agents may be reversed by sufficient doses of adrenergic agonists.
Diabetes Mellitus
Beta-adrenergic blocking agents should be administered with caution in patients subject to spontaneous hypoglycemia or to diabetic patients (especially those with labile diabetes) who are receiving insulin or oral hypoglycemic agents. Beta-adrenergic receptor blocking agents may mask the signs and symptoms of acute hypoglycemia.
Thyrotoxicosis
Beta-adrenergic blocking agents may mask certain clinical signs (e.g., tachycardia) of hyperthyroidism. Patients suspected of developing thyrotoxicosis should be managed carefully to avoid abrupt withdrawal of beta-adrenergic blocking agents that might precipitate a thyroid storm.
PRECAUTIONS
General
Because of potential effects of beta-adrenergic blocking agents on blood pressure and pulse, these agents should be used with caution in patients with cerebrovascular insufficiency. If signs or symptoms suggesting reduced cerebral blood flow develop following initiation of therapy with Timolol maleate ophthalmic gel forming solution, alternative therapy should be considered.
There have been reports of bacterial keratitis associated with the use of multiple-dose containers of topical ophthalmic products. These containers had been inadvertently contaminated by patients who, in most cases, had a concurrent corneal disease or a disruption of the ocular epithelial surface [see PRECAUTIONS, Information for Patients].
Choroidal detachment after filtration procedures has been reported with the administration of aqueous suppressant therapy (e.g. timolol).
Angle-Closure Glaucoma
In patients with angle-closure glaucoma, the immediate objective of treatment is to reopen the angle. This may require constricting the pupil. Timolol maleate has little or no effect on the pupil. Timolol maleate ophthalmic gel forming solution should not be used alone in the treatment of angle-closure glaucoma.
Anaphylaxis
While taking beta-blockers, patients with a history of atopy or a history of severe anaphylactic reactions to a variety of allergens may be more reactive to repeated accidental, diagnostic, or therapeutic challenge with such allergens. Such patients may be unresponsive to the usual doses of epinephrine used to treat anaphylactic reactions.
Muscle Weakness
Beta-adrenergic blockade has been reported to potentiate muscle weakness consistent with certain myasthenic symptoms (e.g., diplopia, ptosis, and generalized weakness). Timolol has been reported rarely to increase muscle weakness in some patients with myasthenia gravis or myasthenic symptoms.
Information for Patients
Patients should be instructed to avoid allowing the tip of the dispensing container to contact the eye or surrounding structures.
Patients should also be instructed that ocular solutions, if handled improperly or if the tip of the dispensing container contacts the eye or surrounding structures, can become contaminated by common bacteria known to cause ocular infections. Serious damage to the eye and subsequent loss of vision may result from using contaminated solutions [see PRECAUTIONS, General].
Patients should also be advised that if they have ocular surgery or develop an intercurrent ocular condition (e.g., trauma or infection), they should immediately seek their physician's advice concerning the continued use of the present multidose container.
Patients should be instructed to invert the closed container and shake once before each use. It is not necessary to shake the container more than once.
Patients requiring concomitant topical ophthalmic medications should be instructed to administer these at least 10 minutes before instilling Timolol maleate ophthalmic gel forming solution.
Patients with bronchial asthma, a history of bronchial asthma, severe chronic obstructive pulmonary disease, sinus bradycardia, second or third degree atrioventricular block, or cardiac failure should be advised not to take this product [see CONTRAINDICATIONS].
Transient blurred vision, generally lasting from 30 seconds to 5 minutes, following instillation, and potential visual disturbances may impair the ability to perform hazardous tasks such as operating machinery or driving a motor vehicle.
Drug Interactions
Beta-Adrenergic Blocking Agents
Patients who are receiving a beta-adrenergic blocking agent orally and Timolol maleate ophthalmic gel forming solution should be observed for potential additive effects of beta-blockade, both systemic and on intraocular pressure. The concomitant use of two topical beta-adrenergic blocking agents is not recommended.
Calcium Antagonists
Caution should be used in the coadministration of beta-adrenergic blocking agents, such as Timolol maleate ophthalmic gel forming solution, and oral or intravenous calcium antagonists because of possible atrioventricular conduction disturbances, left ventricular failure, or hypotension. In patients with impaired cardiac function, coadministration should be avoided.
Catecholamine-Depleting Drugs
Close observation of the patient is recommended when a beta blocker is administered to patients receiving catecholamine-depleting drugs such as reserpine, because of possible additive effects and the production of hypotension and/or marked bradycardia, which may result in vertigo, syncope, or postural hypotension.
Digitalis and Calcium Antagonists
The concomitant use of beta-adrenergic blocking agents with digitalis and calcium antagonists may have additive effects in prolonging atrioventricular conduction time.
CYP2D6 Inhibitors
Potentiated systemic beta-blockade (e.g., decreased heart rate, depression) has been reported during combined treatment with CYP2D6 inhibitors (e.g. quinidine, SSRIs) and timolol.
Clonidine
Oral beta-adrenergic blocking agents may exacerbate the rebound hypertension which can follow the withdrawal of clonidine. There have been no reports of exacerbation of rebound hypertension with ophthalmic timolol maleate.
Injectable Epinephrine
[See PRECAUTIONS, General, Anaphylaxis]
Carcinogenesis, Mutagenesis, Impairment of Fertility
In a two-year study of timolol maleate administered orally to rats, there was a statistically significant increase in the incidence of adrenal pheochromocytomas in male rats administered 300 mg/kg/day (approximately 42,000 times the systemic exposure following the maximum recommended human ophthalmic dose). Similar differences were not observed in rats administered oral doses equivalent to approximately 14,000 times the maximum recommended human ophthalmic dose.
In a lifetime oral study in mice, there were statistically significant increases in the incidence of benign and malignant pulmonary tumors, benign uterine polyps, and mammary adenocarcinomas in female mice at 500 mg/kg/day (approximately 71,000 times the systemic exposure following the maximum recommended human ophthalmic dose), but not at 5 or 50 mg/kg/day (approximately 700 or 7,000, respectively, times the systemic exposure following the maximum recommended human ophthalmic dose). In a subsequent study in female mice, in which post-mortem examinations were limited to the uterus and the lungs, a statistically significant increase in the incidence of pulmonary tumors was again observed at 500 mg/kg/day.
The increased occurrence of mammary adenocarcinomas was associated with elevations in serum prolactin, which occurred in female mice administered oral timolol at 500 mg/kg/day, but not at oral doses of 5 or 50 mg/kg/day. An increased incidence of mammary adenocarcinomas in rodents has been associated with administration of several other therapeutic agents that elevate serum prolactin, but no correlation between serum prolactin levels and mammary tumors has been established in humans. Furthermore, in adult human female subjects who received oral dosages of up to 60 mg of timolol maleate (the maximum recommended human oral dosage), there were no clinically meaningful changes in serum prolactin.
Timolol maleate was devoid of mutagenic potential when tested in vivo (mouse) in the micronucleus test and cytogenetic assay (doses up to 800 mg) and in vitro in a neoplastic cell transformation assay (up to 100 mcg/mL). In Ames tests, the highest concentrations of timolol employed, 5,000 or 10,000 mcg/plate, were associated with statistically significant elevations of revertants observed with tester strain TA 100 (in seven replicate assays), but not in the remaining three strains. In the assays with tester strain TA 100, no consistent dose-response relationship was observed, and the ratio of test to control revertants did not reach 2. A ratio of 2 is usually considered the criterion for a positive Ames test.
Reproduction and fertility studies in rats demonstrated no adverse effect on male or female fertility at doses up to 21,000 times the systemic exposure following the maximum recommended human ophthalmic dose.
Pregnancy
Teratogenic Effects
Teratogenicity studies with timolol in mice, rats, and rabbits at oral doses up to 50 mg/kg/day (7,000 times the systemic exposure following the maximum recommended human ophthalmic dose) demonstrated no evidence of fetal malformations. Although delayed fetal ossification was observed at this dose in rats, there were no adverse effects on postnatal development of offspring. Doses of 1000 mg/kg/day (142,000 times the systemic exposure following the maximum recommended human ophthalmic dose) were maternotoxic in mice and resulted in an increased number of fetal resorptions. Increased fetal resorptions were also seen in rabbits at doses of 14,000 times the systemic exposure following the maximum recommended human ophthalmic dose, in this case without apparent maternotoxicity.
There are no adequate and well-controlled studies in pregnant women. Timolol maleate ophthalmic gel forming solution should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Nursing Mothers
Timolol maleate has been detected in human milk following oral and ophthalmic drug administration. Because of the potential for serious adverse reactions from Timolol maleate ophthalmic gel forming solution in nursing infants, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use
Safety and effectiveness of timolol maleate ophthalmic solution have been established when administered in pediatric patients aged 2 years and older. Use of timolol maleate ophthalmic solution in these children is supported by evidence from adequate and well-controlled studies in children and adults. Safety and efficacy in pediatric patients below the age of 2 years have not been established.
ADVERSE REACTIONS
In clinical trials, transient blurred vision upon instillation of the drop was reported in approximately one in three patients (lasting from 30 seconds to 5 minutes). Less than 1% of patients discontinued from the studies due to blurred vision.
The frequency of patients reporting burning and stinging upon instillation was comparable between Timolol maleate ophthalmic gel forming solution and TIMOPTIC (approximately one in eight patients).
Adverse experiences reported in 1-5% of patients were:
| Ocular: | Pain, conjunctivitis, discharge (e.g., crusting), foreign body sensation, itching and tearing; |
| Systemic: | Headache, dizziness, and upper respiratory infections. |
The following additional adverse experiences have been reported with the ocular administration of this or other timolol maleate formulations:
BODY AS A WHOLE
Asthenia/fatigue, and chest pain.
CARDIOVASCULAR
Bradycardia, arrhythmia, hypotension, hypertension, syncope, heart block, cerebrovascular accident, cerebral ischemia, cardiac failure, worsening of angina pectoris, palpitation, cardiac arrest, pulmonary edema, edema, claudication, Raynaud's phenomenon, and cold hands and feet.
DIGESTIVE
Nausea, diarrhea, dyspepsia, anorexia, and dry mouth.
IMMUNOLOGIC
Systemic lupus erythematosus.
NERVOUS SYSTEM/PSYCHIATRIC
Increase in signs and symptoms of myasthenia gravis, paresthesia, somnolence, insomnia, nightmares, behavioral changes and psychic disturbances including depression, confusion, hallucinations, anxiety, disorientation, nervousness, and memory loss.
SKIN
Alopecia and psoriasiform rash or exacerbation of psoriasis.
HYPERSENSITIVITY
Signs and symptoms of systemic allergic reactions including anaphylaxis, angioedema, urticaria, localized and generalized rash.
RESPIRATORY
Bronchospasm (predominantly in patients with preexisting bronchospastic disease), respiratory failure, dyspnea, nasal congestion, cough and upper respiratory infections.
ENDOCRINE
Masked symptoms of hypoglycemia in diabetic patients [see WARNINGS, Diabetes Mellitus].
SPECIAL SENSES
Signs and symptoms of ocular irritation including blepharitis, keratitis, and dry eyes; ptosis; decreased corneal sensitivity; cystoid macular edema; visual disturbances including refractive changes and diplopia; pseudopemphigoid; choroidal detachment following filtration surgery [see PRECAUTIONS, General]; and tinnitus.
UROGENITAL
Retroperitoneal fibrosis, decreased libido, impotence, and Peyronie's disease.
The following additional adverse effects have been reported in clinical experience with ORAL timolol maleate or other ORAL beta-blocking agents and may be considered potential effects of ophthalmic timolol maleate: Allergic: Erythematous rash, fever combined with aching and sore throat, laryngospasm with respiratory distress; Body as a Whole: Extremity pain, decreased exercise tolerance, weight loss; Cardiovascular: Worsening of arterial insufficiency, vasodilatation; Digestive: Gastrointestinal pain, hepatomegaly, vomiting, mesenteric arterial thrombosis, ischemic colitis; Hematologic: Nonthrombocytopenic purpura, thrombocytopenic purpura, agranulocytosis; Endocrine: Hyperglycemia, hypoglycemia; Skin: Pruritus, skin irritation, increased pigmentation, sweating; Musculoskeletal: Arthralgia; Nervous System/Psychiatric: Vertigo, local weakness, diminished concentration, reversible mental depression progressing to catatonia, an acute reversible syndrome characterized by disorientation for time and place, emotional lability, slightly clouded sensorium, and decreased performance on neuropsychometrics; Respiratory: Rales, bronchial obstruction; Urogenital: Urination difficulties.
To report SUSPECTED ADVERSE REACTIONS, contact Alembic Pharmaceuticals Inc., at 1-866-210-9797 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
OVERDOSAGE
No data are available in regard to human overdosage with or accidental oral ingestion of Timolol maleate ophthalmic gel forming solution. There have been reports of inadvertent overdosage with TIMOPTIC Ophthalmic Solution resulting in systemic effects similar to those seen with systemic beta adrenergic blocking agents such as dizziness, headache, shortness of breath, bradycardia, bronchospasm, and cardiac arrest [see ADVERSE REACTIONS].
Overdosage has been reported with timolol maleate tablets. A 30-year-old female ingested 650 mg of timolol maleate tablets (maximum recommended oral daily dose is 60 mg) and experienced second and third degree heart block. She recovered without treatment but approximately two months later developed irregular heartbeat, hypertension, dizziness, tinnitus, faintness, increased pulse rate, and borderline first degree heart block.
An in vitro hemodialysis study, using 14C timolol added to human plasma or whole blood, showed that timolol was readily dialyzed from these fluids; however, a study of patients with renal failure showed that timolol did not dialyze readily.
DOSAGE AND ADMINISTRATION
Patients should be instructed to invert the closed container and shake once before each use. It is not necessary to shake the container more than once. Other topically applied ophthalmic medications should be administered at least 10 minutes before Timolol maleate ophthalmic gel forming solution [see PRECAUTIONS, Information for Patients and accompanying INSTRUCTIONS FOR USE].
Timolol maleate ophthalmic gel forming solution is available in concentrations of 0.25% and 0.5%. The dose is one drop of Timolol maleate ophthalmic gel forming solution (either 0.25% or 0.5%) in the affected eye(s) once a day.
Because in some patients the pressure-lowering response to Timolol maleate ophthalmic gel forming solution may require a few weeks to stabilize, evaluation should include a determination of intraocular pressure after approximately 4 weeks of treatment with Timolol maleate ophthalmic gel forming solution.
Dosages higher than one drop of 0.5% Timolol maleate ophthalmic gel forming solution once a day have not been studied. If the patient's intraocular pressure is still not at a satisfactory level on this regimen, concomitant therapy can be considered. The concomitant use of two topical beta-adrenergic blocking agents is not recommended [see PRECAUTIONS, Drug Interactions, Beta-adrenergic blocking agents].
When patients have been switched from therapy with TIMOPTIC administered twice daily to Timolol maleate ophthalmic gel forming solution administered once daily, the ocular hypotensive effect has remained consistent.
HOW SUPPLIED
Timolol maleate ophthalmic gel forming solution is a colorless to nearly colorless, slightly opalescent and slightly viscous solution.
Timolol maleate ophthalmic gel forming solution 0.25% timolol equivalent, is supplied in a white low density polyethylene (LDPE) screw neck bottle with a white low density polyethylene (LDPE) nozzle and high density polyethylene (HDPE) yellow screw cap as follows:
NDC 62332-545-05, 5 mL in a 5 mL capacity bottle.
Timolol maleate ophthalmic gel forming solution 0.5% timolol equivalent, is supplied in a white low density polyethylene (LDPE) screw neck bottle with a white low density polyethylene (LDPE) nozzle and high density polyethylene (HDPE) yellow screw cap as follows:
NDC 62332-546-05, 5 mL in a 5 mL capacity bottle.
Storage
Store at 15° to 25°C (59° to 77°F). Avoid Freezing. Protect from light.
TIMOPTIC is trademark of Bausch & Lomb Incorporated or its affiliates.
Manufactured for:
Alembic Pharmaceuticals, Inc.
Bedminster, NJ 07921, USA.
Made in India.
Manufactured by:
Gland Pharma Limited
Hyderabad-500 043, INDIA.
Revised: 06/2021.
Instruction for Use
Timolol Maleate Ophthalmic Gel Forming Solution 0.25% and 0.5%
Read this Instructions for Use that comes with Timolol maleate ophthalmic gel forming solution before you start using it and each time you get a refill. There may be new information. This information does not take the place of talking with your doctor about your medical condition or treatment.
Important information about Timolol maleate ophthalmic gel forming solution:
• Use Timolol maleate ophthalmic gel forming solution exactly as your doctor tells you to use it. Your doctor will tell you how much Timolol maleate ophthalmic gel forming solution to use and when to use it.
• If you use other medicines in your eye, wait at least 10 minutes between using Timolol maleate ophthalmic gel forming solution and your other eye medicines.
• Do not touch your eye or areas around your eye with the tip of the Timolol maleate ophthalmic gel forming solution bottle. You may get bacteria on the tip of the bottle that can cause you to get an eye infection that can lead to serious eye damage or vision loss.
How should I use Timolol maleate ophthalmic gel forming solution?
Step 1. Wash your hands.
Step 2. Turn your closed bottle of Timolol maleate ophthalmic gel forming solution upside down (invert) and shake once.
Step 3. Remove the Timolol maleate ophthalmic gel forming solution cap by turning the cap in the direction of the arrows shown (See Figure A). Put the cap in a clean and dry area. Do not let the tip of the bottle touch your fingers or other surfaces.

Step 4. Hold the bottle between your thumb and index finger with 1 hand. Use the index finger of the other hand to pull down the lower eyelid to form a pocket for the eye drop (See Figure B). Tilt your head backwards.
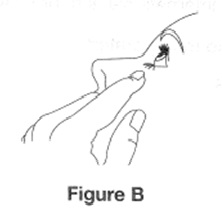
Step 5. Place the tip of the bottle close to your eye. Be careful not to touch your eye with the tip of the bottle. Gently squeeze the bottle and let 1 drop fall into the space between your lower eyelid and your eye (See Figure C). If a drop misses your eye, follow the instructions in steps 4 and 5 again.

Step 6. If your doctor has told you to use Timolol maleate ophthalmic gel forming solution in both eyes, repeat steps 4 and 5 for your other eye.
Step 7. Put the cap back on the bottle and close.
• The Timolol maleate ophthalmic gel forming solution bottle tip is made to give 1 drop at a time. Do not try to make the hole in the tip of your bottle bigger.
• Do not wash the bottle tip.
• After you have used all of your Timolol maleate ophthalmic gel forming solution doses, there will be some Timolol maleate ophthalmic gel forming solution left in the bottle. Do not try to remove the extra Timolol maleate ophthalmic gel forming solution from the bottle. Throw it away.
How should I store Timolol maleate ophthalmic gel forming solution?
• Store Timolol maleate ophthalmic gel forming solution at room temperature between 59⁰F to 77⁰F (15⁰C to 25⁰C) in an upright position.
• Do not freeze Timolol maleate ophthalmic gel forming solution.
• Keep Timolol maleate ophthalmic gel forming solution out of light.
Keep Timolol maleate ophthalmic gel forming solution and all medicines out of the reach of children.
If you would like more information, talk with your doctor. You can ask your pharmacist or doctor for more information about Timolol maleate ophthalmic gel forming solution that is written for health professionals.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
TIMOPTIC is trademark of Bausch & Lomb Incorporated or its affiliates.
Manufactured for:
Alembic Pharmaceuticals, Inc.
Bedminster, NJ 07921, USA.
Made in India.
Manufactured by:
Gland Pharma Limited,
Hyderabad-500 043, INDIA.
Revised: 06/2021.
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
INGREDIENTS AND APPEARANCE
| TIMOLOL MALEATE OPHTHALMIC GEL FORMING SOLUTION, 0.25%
timolol maleate ophthalmic gel forming solution, 0.25% solution/ drops |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| TIMOLOL MALEATE OPHTHALMIC GEL FORMING SOLUTION, 0.5%
timolol maleate ophthalmic gel forming solution, 0.5% solution/ drops |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Alembic Pharmaceuticals Inc. (079288842) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Gland Pharma Limited | 918601238 | MANUFACTURE(62332-545, 62332-546) | |