Search by Drug Name or NDC
NDC 70000-0261-01 Leader Childrens Mucus Relief 5; 100; 2.5 mg/5mL; mg/5mL; mg/5mL Details
Leader Childrens Mucus Relief 5; 100; 2.5 mg/5mL; mg/5mL; mg/5mL
Leader Childrens Mucus Relief is a ORAL SOLUTION in the HUMAN OTC DRUG category. It is labeled and distributed by Cardinal Health 110, LLC. dba Leader. The primary component is DEXTROMETHORPHAN HYDROBROMIDE; GUAIFENESIN; PHENYLEPHRINE HYDROCHLORIDE.
MedlinePlus Drug Summary
Dextromethorphan is used to temporarily relieve cough caused by the common cold, the flu, or other conditions. Dextromethorphan will relieve a cough but will not treat the cause of the cough or speed recovery. Dextromethorphan is in a class of medications called antitussives. It works by decreasing activity in the part of the brain that causes coughing.
Related Packages: 70000-0261-01Last Updated: 12/01/2022
MedLinePlus Full Drug Details: Dextromethorphan
Guaifenesin is used to relieve chest congestion. Guaifenesin may help control symptoms but does not treat the cause of symptoms or speed recovery. Guaifenesin is in a class of medications called expectorants. It works by thinning the mucus in the air passages to make it easier to cough up the mucus and clear the airways.
Related Packages: 70000-0261-01Last Updated: 12/01/2022
MedLinePlus Full Drug Details: Guaifenesin
Phenylephrine is used to relieve nasal discomfort caused by colds, allergies, and hay fever. It is also used to relieve sinus congestion and pressure. Phenylephrine will relieve symptoms but will not treat the cause of the symptoms or speed recovery. Phenylephrine is in a class of medications called nasal decongestants. It works by reducing swelling of the blood vessels in the nasal passages.
Related Packages: 70000-0261-01Last Updated: 12/01/2022
MedLinePlus Full Drug Details: Phenylephrine
Product Information
| NDC | 70000-0261 |
|---|---|
| Product ID | 70000-0261_8912357d-0ea4-4b1e-9713-0c8ffe838cc5 |
| Associated GPIs | 43997303100960 |
| GCN Sequence Number | 066559 |
| GCN Sequence Number Description | guaifen/dextromethorphan/PE LIQUID 5-2.5 MG/5 ORAL |
| HIC3 | B4R |
| HIC3 Description | NON-OPIOID ANTITUSSIVE-DECONGESTANT-EXPECTORANT |
| GCN | 28875 |
| HICL Sequence Number | 000216 |
| HICL Sequence Number Description | GUAIFENESIN/DEXTROMETHORPHAN HBR/PHENYLEPHRINE |
| Brand/Generic | Generic |
| Proprietary Name | Leader Childrens Mucus Relief |
| Proprietary Name Suffix | n/a |
| Non-Proprietary Name | dextromethorphan HBr, guaifenesin, phenylephrine HCl |
| Product Type | HUMAN OTC DRUG |
| Dosage Form | SOLUTION |
| Route | ORAL |
| Active Ingredient Strength | 5; 100; 2.5 |
| Active Ingredient Units | mg/5mL; mg/5mL; mg/5mL |
| Substance Name | DEXTROMETHORPHAN HYDROBROMIDE; GUAIFENESIN; PHENYLEPHRINE HYDROCHLORIDE |
| Labeler Name | Cardinal Health 110, LLC. dba Leader |
| Pharmaceutical Class | Adrenergic alpha1-Agonists [MoA], Sigma-1 Agonist [EPC], Sigma-1 Receptor Agonists [MoA], Uncompetitive N-methyl-D-aspartate Receptor Antagonist [EPC], Uncompetitive NMDA Receptor Antagonists [MoA], alpha-1 Adrenergic Agonist [EPC] |
| DEA Schedule | n/a |
| Marketing Category | OTC MONOGRAPH FINAL |
| Application Number | part341 |
| Listing Certified Through | 2024-12-31 |
Package
Package Images
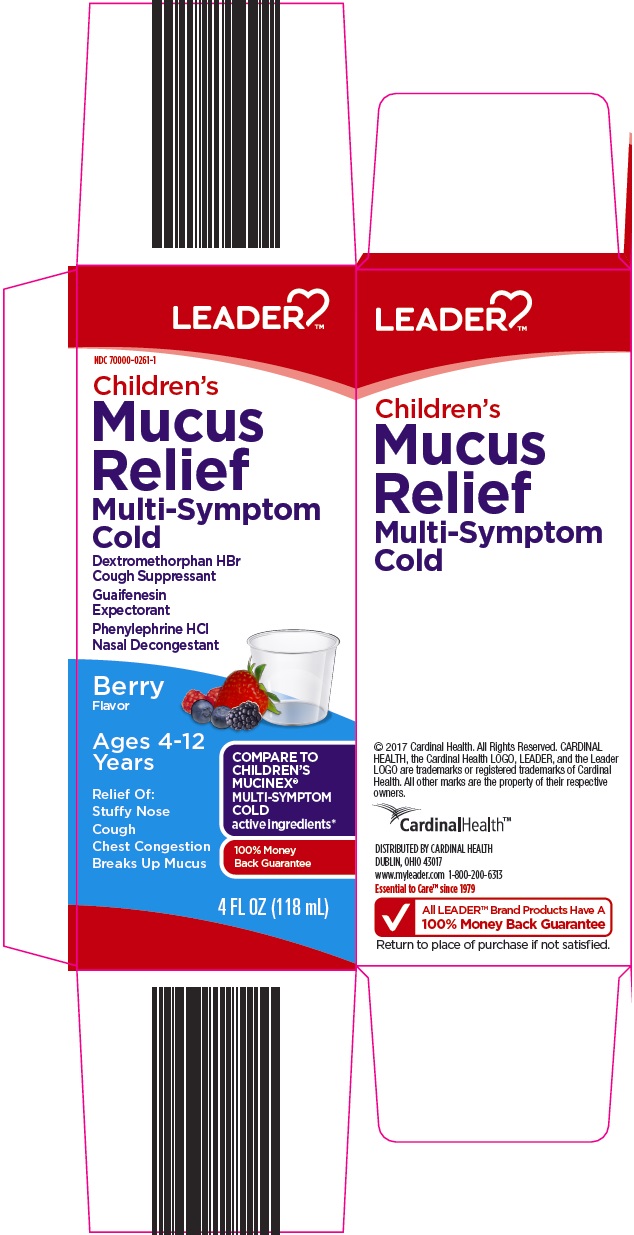

NDC 70000-0261-01 (70000026101)
| NDC Package Code | 70000-0261-1 |
|---|---|
| Billing NDC | 70000026101 |
| Package | 1 BOTTLE in 1 CARTON (70000-0261-1) / 118 mL in 1 BOTTLE |
| Marketing Start Date | 2017-03-22 |
| NDC Exclude Flag | N |
| Pricing Information | |
| Price Per Unit | 0.02739 |
| Pricing Unit | ML |
| Effective Date | 2024-01-17 |
| NDC Description | CHILD MUCUS RELIEF M-S COLD LQ |
| Pharmacy Type Indicator | C/I |
| OTC | Y |
| Explanation Code | 4, 5 |
| Classification for Rate Setting | G |
| As of Date | 2024-02-21 |
Standard Product Labeling (SPL)/Prescribing Information SPL 359f70d5-d3f3-4e0e-a507-5443efa5faf7 Details
Active ingredients (in each 5 mL)
Uses
- •
- helps loosen phlegm (mucus) and thin bronchial secretions to rid the bronchial passageways of bothersome mucus and make coughs more productive
- •
- temporarily relieves:
- •
- cough due to minor throat and bronchial irritation as may occur with the common cold or inhaled irritants
- •
- the intensity of coughing
- •
- the impulse to cough to help your child get to sleep
- •
- nasal congestion due to a cold
- •
- stuffy nose
Warnings
Do not use
in a child who is taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson’s disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your child’s prescription drug contains an MAOI, ask a doctor or pharmacist before giving this product.
Keep out of reach of children.
Directions
- •
- do not take more than 6 doses in any 24-hour period
- •
- measure only with dosing cup provided
- •
- do not use dosing cup with other products
- •
- dose as follows or as directed by a doctor
- •
- mL = milliliter
|
Age |
Dose |
|
children 6 years to under 12 years |
10 mL every 4 hours |
|
children 4 years to under 6 years |
5 mL every 4 hours |
|
children under 4 years |
do not use |
Inactive ingredients
Package/Label Principal Display Panel
Children’s Mucus Relief
Multi-Symptom Cold
Dextromethorphan HBr Cough Suppressant
Guaifenesin Expectorant
Phenylephrine HCl Nasal Decongestant
Berry Flavor
Ages 4-12 Years
COMPARE TO CHILDREN’S MUCINEX® MULTI-SYMPTOM COLD active ingredients
Relief of:
Stuffy Nose
Cough
Chest Congestion
Breaks Up Mucus
4 FL OZ (118 mL)
INGREDIENTS AND APPEARANCE
| LEADER CHILDRENS MUCUS RELIEF
dextromethorphan hbr, guaifenesin, phenylephrine hcl solution |
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
| Labeler - Cardinal Health 110, LLC. dba Leader (063997360) |