Search by Drug Name or NDC
NDC 71930-0051-52 Oxybutynin Chloride 5 mg/1 Details
Oxybutynin Chloride 5 mg/1
Oxybutynin Chloride is a ORAL TABLET in the HUMAN PRESCRIPTION DRUG category. It is labeled and distributed by Eywa Pharma Inc. The primary component is OXYBUTYNIN CHLORIDE.
MedlinePlus Drug Summary
Oxybutynin is used to treat overactive bladder (a condition in which the bladder muscles contract uncontrollably and cause frequent urination, urgent need to urinate, and inability to control urination) in certain adults and children. Oxybutynin is also used as an extended-release tablet to control bladder muscles in adults and children 6 years of age and older with spina bifida (a disability that occurs when the spinal cord does not close properly before birth), or other nervous system conditions that affect the bladder muscles. Oxybutynin is in a class of medications called anticholinergics/antimuscarinics. It works by relaxing the bladder muscles.
Related Packages: 71930-0051-52Last Updated: 12/01/2022
MedLinePlus Full Drug Details: Oxybutynin
Product Information
| NDC | 71930-0051 |
|---|---|
| Product ID | 71930-051_8b369b71-fbec-b41f-e053-2a95a90a4530 |
| Associated GPIs | 54100045200330 |
| GCN Sequence Number | 004929 |
| GCN Sequence Number Description | oxybutynin chloride TABLET 5 MG ORAL |
| HIC3 | R1A |
| HIC3 Description | URINARY TRACT ANTISPASMODIC/ANTIINCONTINENCE AGENT |
| GCN | 19380 |
| HICL Sequence Number | 002048 |
| HICL Sequence Number Description | OXYBUTYNIN CHLORIDE |
| Brand/Generic | Generic |
| Proprietary Name | Oxybutynin Chloride |
| Proprietary Name Suffix | n/a |
| Non-Proprietary Name | Oxybutynin Chloride |
| Product Type | HUMAN PRESCRIPTION DRUG |
| Dosage Form | TABLET |
| Route | ORAL |
| Active Ingredient Strength | 5 |
| Active Ingredient Units | mg/1 |
| Substance Name | OXYBUTYNIN CHLORIDE |
| Labeler Name | Eywa Pharma Inc |
| Pharmaceutical Class | Cholinergic Muscarinic Antagonist [EPC], Cholinergic Muscarinic Antagonists [MoA] |
| DEA Schedule | n/a |
| Marketing Category | ANDA |
| Application Number | ANDA211062 |
| Listing Certified Through | 2022-12-31 |
Package
Package Images

NDC 71930-0051-52 (71930005152)
| NDC Package Code | 71930-051-52 |
|---|---|
| Billing NDC | 71930005152 |
| Package | 500 TABLET in 1 BOTTLE (71930-051-52) |
| Marketing Start Date | 2019-07-15 |
| NDC Exclude Flag | N |
| Pricing Information | |
| Price Per Unit | 0.05289 |
| Pricing Unit | EA |
| Effective Date | 2024-02-21 |
| NDC Description | OXYBUTYNIN 5 MG TABLET |
| Pharmacy Type Indicator | C/I |
| OTC | N |
| Explanation Code | 1 |
| Classification for Rate Setting | G |
| As of Date | 2024-02-21 |
Standard Product Labeling (SPL)/Prescribing Information SPL 8b369b71-fbeb-b41f-e053-2a95a90a4530 Details
DESCRIPTION
Each scored oxybutynin chloride tablet contains 5 mg of oxybutynin chloride. Chemically, oxybutynin chloride is d,l (racemic) 4-diethylamino-2-butynyl phenylcyclohexylglycolate hydrochloride. The empirical formula of oxybutynin chloride is C 22H 31NO 3•HCl. The structural formula appears below:
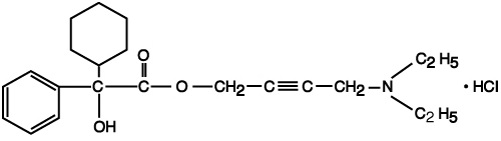
Oxybutynin chloride is a white crystalline solid with a molecular weight of 393.95. It is readily soluble in water and acids, but relatively insoluble in alkalis.
Oxybutynin Chloride Tablets also contain: magnesium stearate, microcrystalline cellulose, and pregelatinized starch.
Oxybutynin Chloride Tablets are for oral administration.
Therapeutic Category: Antispasmodic, anticholinergic.
Meets USP Dissolution Test 2.
CLINICAL PHARMACOLOGY
Oxybutynin chloride exerts a direct antispasmodic effect on smooth muscle and inhibits the muscarinic action of acetylcholine on smooth muscle. Oxybutynin chloride exhibits only one-fifth of the anticholinergic activity of atropine on the rabbit detrusor muscle, but four to ten times the antispasmodic activity. No blocking effects occur at skeletal neuromuscular junctions or autonomic ganglia (antinicotinic effects).
Oxybutynin chloride relaxes bladder smooth muscle. In patients with conditions characterized by involuntary bladder contractions, cystometric studies have demonstrated that oxybutynin chloride increases bladder (vesical) capacity, diminishes the frequency of uninhibited contractions of the detrusor muscle, and delays the initial desire to void. Oxybutynin chloride thus decreases urgency and the frequency of both incontinent episodes and voluntary urination.
Antimuscarinic activity resides predominately in the R-isomer. A metabolite, desethyloxybutynin, has pharmacological activity similar to that of oxybutynin in in vitro studies.
PHARMACOKINETICS
Absorption
Following oral administration of oxybutynin chloride, oxybutynin is rapidly absorbed achieving C max within an hour, following which plasma concentration decreases with an effective half-life of approximately 2 to 3 hours. The absolute bioavailability of oxybutynin is reported to be about 6% (range 1.6 to 10.9%) for the tablets. Wide interindividual variation in pharmacokinetic parameters is evident following oral administration of oxybutynin.
The mean pharmacokinetic parameters for R- and S-oxybutynin are summarized in Table 1. The plasma concentration-time profiles for R- and S-oxybutynin are similar in shape; Figure 1 shows the profile for R-oxybutynin.
|
Table 1 Mean (SD) R- and S-Oxybutynin Pharmacokinetic Parameters Following Three Doses of Oxybutynin Chloride 5 mg Administered every 8 Hours (n=23) |
||
|
Parameters (units) |
R-Oxybutynin |
S-Oxybutynin |
|
C max (ng/mL) |
3.6 (2.2) |
7.8 (4.1) |
|
T max (h) |
0.89 (0.34) |
0.65 (0.32) |
|
AUC t(ng•h/mL) |
22.6 (11.3) |
35.0 (17.3) |
|
AUC inf (ng•h/mL) |
24.3 (12.3) |
37.3 (18.7) |
Figure 1. Mean R-oxybutynin plasma concentrations following three doses of oxybutynin chloride 5 mg administered every 8 hours for 1 day in 23 healthy adult volunteers

Oxybutynin chloride steady-state pharmacokinetics were also studied in 11 pediatric patients with detrusor overactivity associated with a neurological condition (e.g., spina bifida). These pediatric patients were on oxybutynin chloride tablets with total daily dose ranging from 7.5 mg to 15 mg (0.22 to 0.53 mg/kg). Overall, most patients (86.9%) were taking a total daily oxybutynin chloride dose between 10 mg and 15 mg. Sparse sampling technique was used to obtain serum samples. When all available data are normalized to an equivalent of 5 mg twice daily oxybutynin chloride, the mean pharmacokinetic parameters derived for R- and S-oxybutynin and R- and S-desethyloxybutynin are summarized in Table 2. The plasma-time concentration profiles for R- and S-oxybutynin are similar in shape; Figure 2 shows the profile for R-oxybutynin when all available data are normalized to an equivalent of 5 mg twice daily.
|
Table 2 Mean ± SD R- and S-Oxybutynin and R- and S-Desethyloxybutynin Pharmacokinetic Parameters In Children Aged 5 to 15 Following Administration of 7.5 mg to 15 mg Total Daily Dose of Oxybutynin Chloride Tablets (N=11) |
||||
|
All Available Data Normalized to an Equivalent of Oxybutynin Chloride Tablets 5 mg BID or TID at Steady State |
||||
|
R-Oxybutynin |
S-Oxybutynin |
R-Desethyloxybutynin |
S-Desethyloxybutynin |
|
|
C max* (ng/mL) |
6.1 ± 3.2 |
10.1 ± 7.5 |
55.4 ± 17.9 |
28.2 ± 10.0 |
|
T max (hr) |
1.0 |
1.0 |
2.0 |
2.0 |
|
AUC† (ng∙hr/mL) |
19.8 ± 7.4 |
28.4 ± 12.7 |
238.8 ± 77.6 |
119.5 ± 50.7 |
* Reflects C max for pooled data
† AUC 0-end of dosing interval
Figure 2. Mean steady-state (±SD) R-oxybutynin plasma concentrations following administration of total daily oxybutynin chloride tablet dose of 7.5 mg to 15 mg (0.22 mg/kg to 0.53 mg/kg) in children 5 to 15 years of age. – Plot represents all available data normalized to the equivalent of oxybutynin chloride 5 mg BID or TID at steady state

Food Effects
Data in the literature suggests that oxybutynin solution co-administered with food resulted in a slight delay in absorption and an increase in its bioavailability by 25% (n=18). 1
Distribution
Oxybutynin is widely distributed in body tissues following systemic absorption. The volume of distribution is 193 L after intravenous administration of 5 mg oxybutynin chloride. Both enantiomers of oxybutynin are highly bound (> 99%) to plasma proteins. Both enantiomers of desethyloxybutynin are also highly bound (> 97%) to plasma proteins. The major binding protein is alpha-1 acid glycoprotein.
Metabolism
Oxybutynin is metabolized primarily by the cytochrome P450 enzyme systems, particularly CYP3A4 found mostly in the liver and gut wall. Its metabolic products include phenylcyclohexylglycolic acid, which is pharmacologically inactive, and desethyloxybutynin, which is pharmacologically active.
Excretion
Oxybutynin is extensively metabolized by the liver, with less than 0.1% of the administered dose excreted unchanged in the urine. Also, less than 0.1% of the administered dose is excreted as the metabolite desethyloxybutynin.
CLINICAL STUDIES
INDICATIONS AND USAGE
CONTRAINDICATIONS
Oxybutynin chloride is contraindicated in patients with urinary retention, gastric retention and other severe decreased gastrointestinal motility conditions, uncontrolled narrow-angle glaucoma and in patients who are at risk for these conditions.
Oxybutynin chloride is also contraindicated in patients who have demonstrated hypersensitivity to the drug substance or other components of the product.
WARNINGS
Angioedema of the face, lips, tongue and/or larynx has been reported with oxybutynin. In some cases, angioedema occurred after the first dose. Angioedema associated with upper airway swelling may be life-threatening. If involvement of the tongue, hypopharynx, or larynx occurs, oxybutynin should be promptly discontinued and appropriate therapy and/or measures necessary to ensure a patent airway should be promptly provided.
PRECAUTIONS
Central Nervous System Effects
Oxybutynin is associated with anticholinergic central nervous system (CNS) effects (see ADVERSE REACTIONS). A variety of CNS anticholinergic effects have been reported, including hallucinations, agitation, confusion and somnolence. Patients should be monitored for signs of anticholinergic CNS effects, particularly in the first few months after beginning treatment or increasing the dose. If a patient experiences anticholinergic CNS effects, dose reduction or drug discontinuation should be considered.
Oxybutynin chloride should be used with caution in patients with pre-existing dementia treated with cholinesterase inhibitors due to the risk of aggravation of symptoms.
Oxybutynin chloride should be used with caution in patients with Parkinson’s disease due to the risk of aggravation of symptoms.
General
Oxybutynin chloride should be used with caution in the frail elderly, in patients with hepatic or renal impairment, and in patients with myasthenia gravis.
Oxybutynin chloride may aggravate the symptoms of hyperthyroidism, coronary heart disease, congestive heart failure, cardiac arrhythmias, hiatal hernia, tachycardia, hypertension, myasthenia gravis, and prostatic hypertrophy.
Urinary Retention
Oxybutynin chloride should be administered with caution to patients with clinically significant bladder outflow obstruction because of the risk of urinary retention (see CONTRAINDICATIONS).
Gastrointestinal Disorders
Oxybutynin chloride should be used with caution in patients with autonomic neuropathy due to the risk of aggravation of symptoms of decreased gastrointestinal motility.
Oxybutynin chloride should be administered with caution to patients with gastrointestinal obstructive disorders because of the risk of gastric retention (see CONTRAINDICATIONS).
Administration of oxybutynin chloride to patients with ulcerative colitis may suppress intestinal motility to the point of producing a paralytic ileus and precipitate or aggravate toxic megacolon, a serious complication of the disease.
Oxybutynin chloride, like other anticholinergic drugs, may decrease gastrointestinal motility and should be used with caution in patients with conditions such as ulcerative colitis, and intestinal atony.
Oxybutynin chloride should be used with caution in patients who have gastroesophageal reflux and/or who are concurrently taking drugs (such as bisphosphonates) that can cause or exacerbate esophagitis.
Information for Patients
Patients should be informed that oxybutynin may produce angioedema that could result in life-threatening airway obstruction. Patients should be advised to promptly discontinue oxybutynin therapy and seek immediate medical attention if they experience edema of the tongue, edema of the laryngopharynx, or difficulty breathing.
Patients should be informed that heat prostration (fever and heat stroke due to decreased sweating) can occur when anticholinergics such as oxybutynin chloride are administered in the presence of high environmental temperature.
Because anticholinergic agents such as oxybutynin may produce drowsiness (somnolence), or blurred vision, patients should be advised to exercise caution.
Patients should be informed that alcohol may enhance the drowsiness caused by anticholinergic agents such as oxybutynin.
Drug Interactions
The concomitant use of oxybutynin with other anticholinergic drugs or with other agents which produce dry mouth, constipation, somnolence (drowsiness), and/or other anticholinergic-like effects may increase the frequency and/or severity of such effects.
Anticholinergic agents may potentially alter the absorption of some concomitantly administered drugs due to anticholinergic effects on gastrointestinal motility. This may be of concern for drugs with a narrow therapeutic index. Anticholinergic agents may also antagonize the effects of prokinetic agents, such as metoclopramide.
Mean oxybutynin chloride plasma concentrations were approximately 3 to 4 fold higher when oxybutynin chloride was administered with ketoconazole, a potent CYP3A4 inhibitor.
Other inhibitors of the cytochrome P450 3A4 enzyme system, such as antimycotic agents (e.g., itraconazole and miconazole) or macrolide antibiotics (e.g., erythromycin and clarithromycin), may alter oxybutynin mean pharmacokinetic parameters (i.e., C max and AUC). The clinical relevance of such potential interactions is not known. Caution should be used when such drugs are co-administered.
Carcinogenesis, Mutagenesis, Impairment of Fertility
A 24-month study in rats at dosages of oxybutynin chloride of 20, 80, and 160 mg/kg/day showed no evidence of carcinogenicity. These doses are approximately 6, 25, and 50 times the maximum human exposure, based on surface area.
Oxybutynin chloride showed no increase of mutagenic activity when tested in Schizosaccharomyces pompholiciformis, Saccharomyces cerevisiae and Salmonella typhimurium test systems.
Reproduction studies using oxybutynin chloride in the hamster, rabbit, rat, and mouse have shown no definite evidence of impaired fertility.
Pregnancy
Category B.
Reproduction studies using oxybutynin chloride in the hamster, rabbit, rat, and mouse have shown no definite evidence of impaired fertility or harm to the animal fetus. The safety of oxybutynin chloride administered to women who are or who may become pregnant has not been established. Therefore, oxybutynin chloride should not be given to pregnant women unless, in the judgment of the physician, the probable clinical benefits outweigh the possible hazards.
Nursing Mothers
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when oxybutynin chloride is administered to a nursing woman.
Pediatric Use
The safety and efficacy of oxybutynin chloride administration have been demonstrated for pediatric patients 5 years of age and older (see DOSAGE AND ADMINISTRATION).
The safety and efficacy of oxybutynin chloride tablets were studied in 30 children in a 24-week, open-label trial. Patients were aged 5 to 15 years, all had symptoms of detrusor overactivity in association with a neurological condition (e.g., spina bifida), all used clean intermittent catheterization, and all were current users of oxybutynin chloride. Study results demonstrated that the administration of oxybutynin chloride was associated with improvement in clinical and urodynamic parameters.
At total daily doses ranging from 5 mg to 15 mg, treatment with oxybutynin chloride tablets was associated with an increase from baseline in mean urine volume per catheterization from 122 mL to 145 mL, an increase from baseline in mean urine volume after morning awakening from 148 mL to 168 mL, and an increase from baseline in the mean percentage of catheterizations without a leaking episode from 43% to 61%. Urodynamic results in these patients were consistent with the clinical results. Treatment with oxybutynin chloride tablets was associated with an increase from baseline in maximum cystometric capacity from 230 mL to 279 mL, a decrease from baseline in mean detrusor pressure at maximum cystometric capacity from 36 cm H 2O to 33 cm H 2O, and a reduction in the percentage of patients demonstrating uninhibited detrusor contractions (of at least 15 cm H 2O) from 39% to 20%.
As there is insufficient clinical data for pediatric populations under age 5, oxybutynin chloride is not recommended for this age group.
Geriatric Use
Clinical studies of oxybutynin chloride did not include sufficient numbers of subjects age 65 and over to determine whether they respond differently from younger patients. Other reported clinical experience has not identified differences in responses between healthy elderly and younger patients; however, a lower initial starting dose of 2.5 mg given 2 or 3 times a day has been recommended for the frail elderly due to a prolongation of the elimination half-life from 2 to 3 hours to 5 hours. 2,3,4 In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
ADVERSE REACTIONS
The safety and efficacy of oxybutynin chloride was evaluated in a total of 199 patients in three clinical trials. These participants were treated with oxybutynin chloride 5 to 20 mg/day for up to 6 weeks. Table 3 shows the incidence of adverse events judged by investigators to be at least possibly related to treatment and reported by at least 5% of patients.
|
Table 3 Incidence (%) of Adverse Events Reported by ≥ 5% of Patients Using Oxybutynin Chloride (5 to 20 mg/day) |
||
|
Body System |
Adverse Event |
Oxybutynin
|
|
Infections and Infestations |
Urinary tract infection |
6.5% |
|
Psychiatric Disorders |
Insomnia |
5.5% |
|
Nervousness |
6.5% |
|
|
Nervous System Disorders |
Dizziness |
16.6% |
|
Somnolence |
14.0% |
|
|
Headache |
7.5% |
|
|
Eye Disorders |
Blurred vision |
9.6% |
|
Gastrointestinal Disorders |
Dry mouth |
71.4% |
|
Constipation |
15.1% |
|
|
Nausea |
11.6% |
|
|
Dyspepsia |
6.0% |
|
|
Renal and Urinary Disorders |
Urinary Hesitation |
8.5% |
|
Urinary Retention |
6.0% |
|
The most common adverse events reported by patients receiving oxybutynin chloride 5 to 20 mg/day were the expected side effects of anticholinergic agents. The incidence of dry mouth was dose-related.
In addition, the following adverse events were reported by 1 to < 5% of patients using oxybutynin chloride (5 to 20 mg/day) in all studies. Infections and Infestations: nasopharyngitis, upper respiratory tract infection, bronchitis, cystitis, fungal infection; Metabolism and Nutrition Disorders: fluid retention; Psychiatric Disorders: confusional state; Nervous System Disorders: dysgeusia, sinus headache; Eye Disorders: keratoconjunctivitis sicca, eye irritation; Cardiac Disorders: palpitations, sinus arrhythmia; Vascular Disorders: flushing; Respiratory, Thoracic and Mediastinal Disorders: nasal dryness, cough, pharyngolaryngeal pain, dry throat, sinus congestion, hoarseness, asthma, nasal congestion; Gastrointestinal Disorders: diarrhea, abdominal pain, loose stools, flatulence, vomiting, abdominal pain upper, dysphagia, aptyalism, eructation, tongue coated; Skin and Subcutaneous Tissue Disorders: dry skin, pruritis; Musculoskeletal and Connective Tissue Disorders: back pain, arthralgia, pain in extremity, flank pain; Renal and Urinary Disorders: dysuria, pollakiuria; General Disorders and Administration Site Conditions: fatigue, edema peripheral, asthenia, pain, thirst, edema; Investigations: blood pressure increased, blood glucose increased, blood pressure decreased; Injury, Poisoning, and Procedural Complications: fall.
Postmarketing Surveillance
Because postmarketing adverse events are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. The following additional adverse events have been reported from worldwide postmarketing experience with oral oxybutynin chloride: Psychiatric Disorders: psychotic disorder, agitation, hallucinations, memory impairment; Nervous System Disorders: convulsions; Eye Disorders: cycloplegia, mydriasis, glaucoma; Cardiac Disorders: tachycardia, QT interval prolongation, chest discomfort; Gastrointestinal Disorders: decreased gastrointestinal motility, frequent bowel movements; Skin and Subcutaneous Tissue Disorders: rash, decreased sweating; Renal and Urinary Disorders: impotence; Reproductive System and Breast Disorders: suppression of lactation; General Disorders and Administration Site Conditions: hypersensitivity reactions, including angioedema with airway obstruction, urticaria, and face edema; rare anaphylactic reactions requiring hospitalization for emergency treatment;. Metabolism and Nutrition Disorders: anorexia; Respiratory, Thoracic and Mediastinal Disorders: dysphonia.
OVERDOSAGE
Treatment should be symptomatic and supportive. Activated charcoal as well as a cathartic may be administered.
Overdosage with oxybutynin chloride has been associated with anticholinergic effects including central nervous system excitation (e.g., restlessness, tremor, irritability, convulsions, delirium, hallucinations), flushing, fever, dehydration, cardiac arrhythmia, vomiting, and urinary retention. Other symptoms may include hypotension or hypertension, respiratory failure, paralysis, and coma.
Ingestion of 100 mg oxybutynin chloride in association with alcohol has been reported in a 13 year old boy who experienced memory loss, and a 34 year old woman who developed stupor, followed by disorientation and agitation on awakening, dilated pupils, dry skin, cardiac arrhythmia, and retention of urine. Both patients fully recovered with symptomatic treatment.
DOSAGE AND ADMINISTRATION
Adults
The usual dose is one 5-mg tablet two to three times a day. The maximum recommended dose is one 5-mg tablet four times a day. A lower starting dose of 2.5 mg two or three times a day is recommended for the frail elderly.
Pediatric patients over 5 years of age
The usual dose is one 5-mg tablet two times a day. The maximum recommended dose is one 5-mg tablet three times a day.
HOW SUPPLIED
Oxybutynin Chloride Tablets USP, 5mg are white to off white, round biconvex uncoated, scored tablet debossed “ET” on one side of break line and “33”on other side, and plain on the other side.
The tablets are supplied as follows:
- · Bottles of 100: NDC 71930-051-12
- · Bottles of 500: NDC 71930-051-52
- · Bottles of 1000: NDC 71930-051-13
Pharmacist: Dispense in a tight, light-resistant container as defined in the USP/NF.
Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature].
REFERENCES SECTION
1 Yong C et al. Effect of Food on the Pharmacokinetics of Oxybutynin in normal subjects. Pharm Res. 1991; 8 (Suppl.): S-320.
2 Hughes KM et al. Measurement of oxybutynin and its N-desethyl metabolite in plasma, and its application to pharmacokinetic studies in young, elderly and frail elderly volunteers. Xenobiotica. 1992; 22 (7): 859–869.
3 Ouslander J et al. Pharmacokinetics and Clinical Effects of Oxybutynin in Geriatric Patients. J. Urol. 1988; 140: 47–50.
4 Yarker Y et al. Oxybutynin: A review of its Pharmacodynamic and Pharmacokinetic Properties, and its Therapeutic Use in Detrusor Instability. Drugs & Aging. 1995; 6 (3): 243–262.
Manufactured for:
Eywa Pharma Inc
2 Research Way, Floor 3
Princeton, NJ 08540
Rev. 05/2019
OXYBUTYNIN CHLORIDE TABLETS, USP 5mg
INGREDIENTS AND APPEARANCE
| OXYBUTYNIN CHLORIDE
oxybutynin chloride tablet |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Labeler - Eywa Pharma Inc (080465609) |