Search by Drug Name or NDC
NDC 00280-6060-20 Aleve 220 mg/1 Details
Aleve 220 mg/1
Aleve is a ORAL TABLET in the HUMAN OTC DRUG category. It is labeled and distributed by Bayer HealthCare LLC.. The primary component is NAPROXEN SODIUM.
MedlinePlus Drug Summary
Prescription naproxen is used to relieve pain, tenderness, swelling, and stiffness caused by osteoarthritis (arthritis caused by a breakdown of the lining of the joints), rheumatoid arthritis (arthritis caused by swelling of the lining of the joints), juvenile arthritis (a form of joint disease in children), and ankylosing spondylitis (arthritis that mainly affects the spine). Prescription naproxen tablets, extended-release tablets, and suspension are also used to relieve shoulder pain caused by bursitis (inflammation of a fluid-filled sac in the shoulder joint), tendinitis (inflammation of the tissue that connects muscle to bone), gouty arthritis (attacks of joint pain caused by a build-up of certain substances in the joints), and pain from other causes, including menstrual pain (pain that happens before or during a menstrual period). Nonprescription naproxen is used to reduce fever and to relieve mild pain from headaches, muscle aches, arthritis, menstrual periods, the common cold, toothaches, and backaches. Naproxen is in a class of medications called NSAIDs. It works by stopping the body's production of a substance that causes pain, fever, and inflammation.
Related Packages: 00280-6060-20Last Updated: 11/30/2022
MedLinePlus Full Drug Details: Naproxen
Product Information
| NDC | 00280-6060 |
|---|---|
| Product ID | 0280-6060_ebcaf0ca-b283-5de8-e053-2a95a90ae5fc |
| Associated GPIs | |
| GCN Sequence Number | n/a |
| GCN Sequence Number Description | n/a |
| HIC3 | n/a |
| HIC3 Description | n/a |
| GCN | n/a |
| HICL Sequence Number | n/a |
| HICL Sequence Number Description | n/a |
| Brand/Generic | n/a |
| Proprietary Name | Aleve |
| Proprietary Name Suffix | Easy Open Arthritis Cap |
| Non-Proprietary Name | NAPROXEN SODIUM |
| Product Type | HUMAN OTC DRUG |
| Dosage Form | TABLET |
| Route | ORAL |
| Active Ingredient Strength | 220 |
| Active Ingredient Units | mg/1 |
| Substance Name | NAPROXEN SODIUM |
| Labeler Name | Bayer HealthCare LLC. |
| Pharmaceutical Class | Anti-Inflammatory Agents, Non-Steroidal [CS], Cyclooxygenase Inhibitors [MoA], Nonsteroidal Anti-inflammatory Drug [EPC] |
| DEA Schedule | n/a |
| Marketing Category | NDA |
| Application Number | NDA020204 |
| Listing Certified Through | 2024-12-31 |
Package
Package Images

NDC 00280-6060-20 (00280606020)
| NDC Package Code | 0280-6060-20 |
|---|---|
| Billing NDC | 00280606020 |
| Package | 200 TABLET in 1 BOTTLE (0280-6060-20) |
| Marketing Start Date | 2014-09-09 |
| NDC Exclude Flag | N |
| Pricing Information | N/A |
Standard Product Labeling (SPL)/Prescribing Information SPL 3c0ddd25-5a9a-4628-a971-65fe2a9775b5 Details
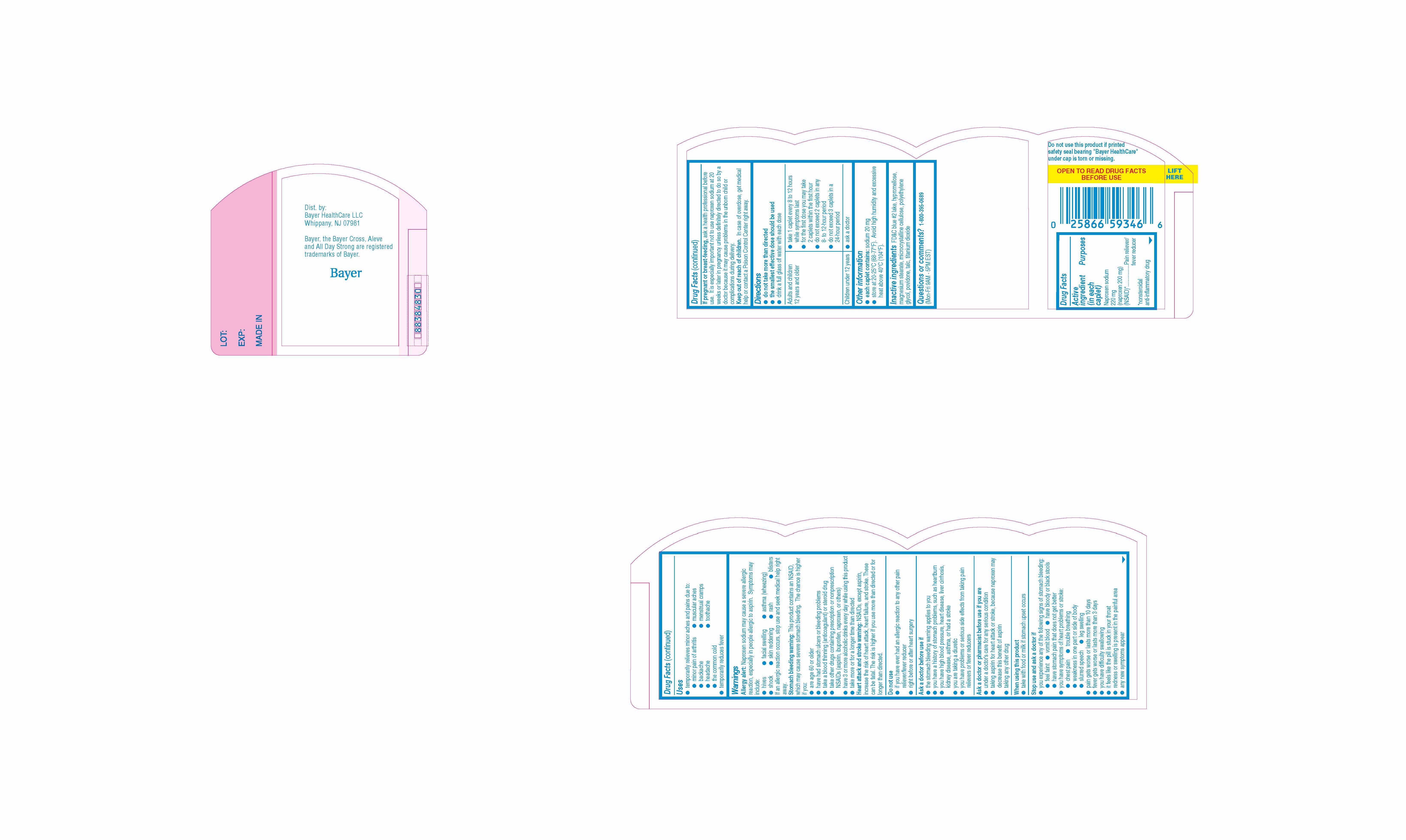
Active ingredient (in each tablet)
Uses
SPL UNCLASSIFIED SECTION
Allergy alert
Naproxen sodium may cause a severe allergic reaction, especially in people allergic to aspirin. Symptoms may include:
- hives
- facial swelling
- asthma (wheezing)
- shock
- skin reddening
- rash
- blisters
If an allergic reaction occurs, stop use and seek medical help right away.
Stomach bleeding warningThis product contains an NSAID, which may cause severe stomach bleeding. The chance is higher if you:
- are age 60 or older
- have had stomach ulcers or bleeding problems
- take a blood thinning (anticoagulant) or steroid drug
- take other drugs containing prescription or nonprescription NSAIDs (aspirin, ibuprofen, naproxen, or others)
- have 3 or more alcoholic drinks every day while using this product
- take more or for a longer time than directed
Heart attack warning
NSAIDs, except aspirin, increase the risk of heart attack, heart failure, and stroke. These can be fatal. The risk is higher if you use more than directed or for longer than directed.
Do not use
- if you have ever had an allergic reaction to any other pain reliever/fever reducer
- right before or after heart surgery
Ask a doctor before use if
- the stomach bleeding warning applies to you
- you have a history of stomach problems, such as heartburn
- you have high blood pressure, heart disease, liver cirrhosis, kidney disease, asthma, or had a stroke
- you are taking a diuretic
- you have problems or serious side effects from taking pain relievers or fever reducers
Ask a doctor or pharmacist before use if you are
- under a doctor's care for any serious condition
- taking aspirin for heart attack or stroke, because naproxen may decrease this benefit of aspirin
- taking any other drug
Stop use and ask a doctor if
- you experience any of the following signs of stomach bleeding:
- feel faint
- vomit blood
- have bloody or black stools
- have stomach pain that does not get better
- you have symptoms of heart problems or stroke:
- chest pain
- trouble breathing
- weakness in one part or side of body
- slurred speech
- leg swelling
- pain gets worse or lasts more than 10 days
- fever gets worse or lasts more than 3 days
- you have difficulty swallowing
- it feels like the pill is stuck in your throat
- redness or swelling is present in the painful area
- any new symptoms appear
Directions
Directions
· do not take more than directed
· the smallest effective dose should be used
· drink a full glass of water with each dose
| Adults and children 12 years and older |
|
| Children under 12 years of age |
|
Other information
Inactive ingredients
PRINCIPAL DISPLAY PANEL - 320 Tablet Bottle Label
INGREDIENTS AND APPEARANCE
| ALEVE
EASY OPEN ARTHRITIS CAP
naproxen sodium tablet |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Labeler - Bayer HealthCare LLC. (112117283) |