Search by Drug Name or NDC
NDC 00536-1333-21 Guaifenesin D 600; 60 mg/1; mg/1 Details
Guaifenesin D 600; 60 mg/1; mg/1
Guaifenesin D is a ORAL TABLET, EXTENDED RELEASE in the HUMAN OTC DRUG category. It is labeled and distributed by RUGBY LABORATORIES, INC.. The primary component is GUAIFENESIN; PSEUDOEPHEDRINE HYDROCHLORIDE.
MedlinePlus Drug Summary
Guaifenesin is used to relieve chest congestion. Guaifenesin may help control symptoms but does not treat the cause of symptoms or speed recovery. Guaifenesin is in a class of medications called expectorants. It works by thinning the mucus in the air passages to make it easier to cough up the mucus and clear the airways.
Related Packages: 00536-1333-21Last Updated: 11/30/2022
MedLinePlus Full Drug Details: Guaifenesin
Pseudoephedrine is used to relieve nasal congestion caused by colds, allergies, and hay fever. It is also used to temporarily relieve sinus congestion and pressure. Pseudoephedrine will relieve symptoms but will not treat the cause of the symptoms or speed recovery. Pseudoephedrine is in a class of medications called nasal decongestants. It works by causing narrowing of the blood vessels in the nasal passages.
Related Packages: 00536-1333-21Last Updated: 11/30/2022
MedLinePlus Full Drug Details: Pseudoephedrine
Product Information
| NDC | 00536-1333 |
|---|---|
| Product ID | 0536-1333_4f4c9058-ec1c-48d5-81df-56188b7ae803 |
| Associated GPIs | 43996202307435 |
| GCN Sequence Number | 012073 |
| GCN Sequence Number Description | guaifenesin/pseudoephedrne HCl TAB ER 12H 600MG-60MG ORAL |
| HIC3 | B4W |
| HIC3 Description | DECONGESTANT-EXPECTORANT COMBINATIONS |
| GCN | 54980 |
| HICL Sequence Number | 000270 |
| HICL Sequence Number Description | GUAIFENESIN/PSEUDOEPHEDRINE HCL |
| Brand/Generic | Generic |
| Proprietary Name | Guaifenesin D |
| Proprietary Name Suffix | n/a |
| Non-Proprietary Name | Guaifenesin and Pseudoephedrine Hydrochloride |
| Product Type | HUMAN OTC DRUG |
| Dosage Form | TABLET, EXTENDED RELEASE |
| Route | ORAL |
| Active Ingredient Strength | 600; 60 |
| Active Ingredient Units | mg/1; mg/1 |
| Substance Name | GUAIFENESIN; PSEUDOEPHEDRINE HYDROCHLORIDE |
| Labeler Name | RUGBY LABORATORIES, INC. |
| Pharmaceutical Class | Adrenergic alpha-Agonists [MoA], Decreased Respiratory Secretion Viscosity [PE], Expectorant [EPC], Increased Respiratory Secretions [PE], alpha-Adrenergic Agonist [EPC] |
| DEA Schedule | n/a |
| Marketing Category | ANDA |
| Application Number | ANDA212542 |
| Listing Certified Through | 2024-12-31 |
Package
Package Images
NDC 00536-1333-21 (00536133321)
| NDC Package Code | 0536-1333-21 |
|---|---|
| Billing NDC | 00536133321 |
| Package | 1 BLISTER PACK in 1 CARTON (0536-1333-21) / 18 TABLET, EXTENDED RELEASE in 1 BLISTER PACK |
| Marketing Start Date | 2022-04-04 |
| NDC Exclude Flag | N |
| Pricing Information | |
| Price Per Unit | 0.35738 |
| Pricing Unit | EA |
| Effective Date | 2024-02-21 |
| NDC Description | GUAIFENESIN-PSE ER 600-60 MG |
| Pharmacy Type Indicator | C/I |
| OTC | Y |
| Explanation Code | 1 |
| Classification for Rate Setting | G |
| As of Date | 2024-02-21 |
Standard Product Labeling (SPL)/Prescribing Information SPL 997946e6-eff0-4c15-8694-020a19c2d336 Details
SPL UNCLASSIFIED SECTION
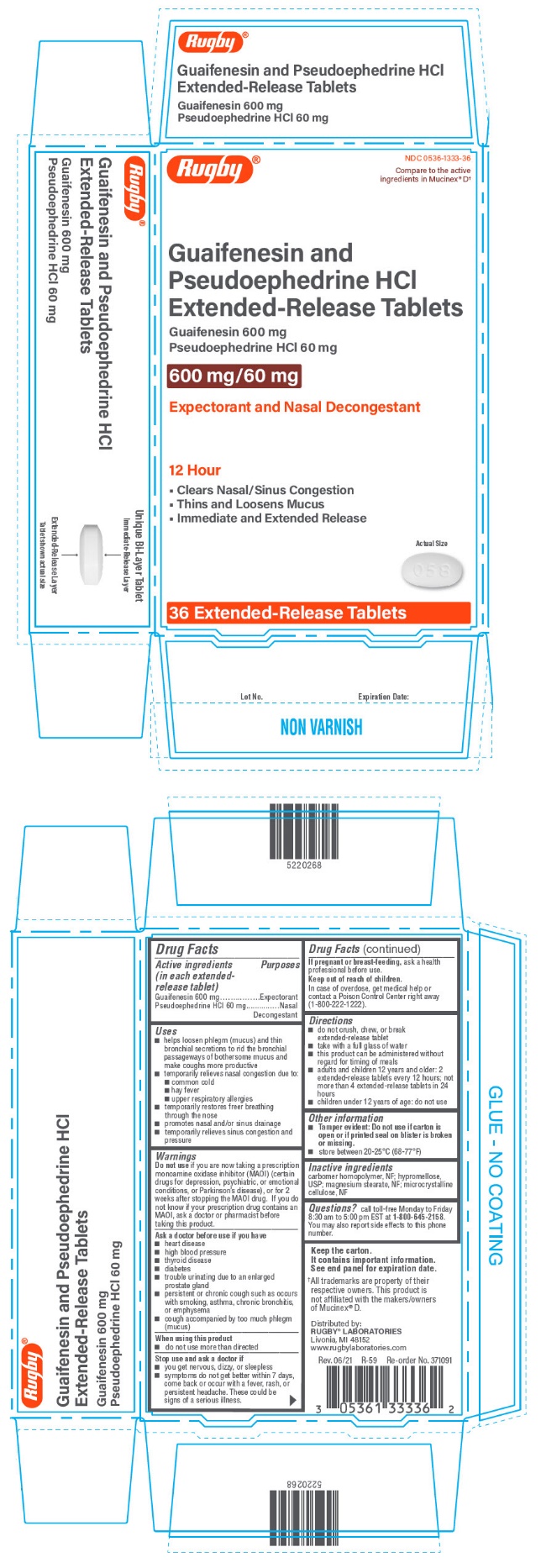
Uses
- •
- helps loosen phlegm (mucus) and thin bronchial secretions to rid the bronchial passageways of bothersome mucus and make coughs more productive
- •
- temporarily relieves nasal congestion due to:
- •
- common cold
- •
- hay fever
- •
- upper respiratory allergies
- •
- temporarily restores freer breathing through the nose
- •
- promotes nasal and/or sinus drainage
- •
- temporarily relieves sinus congestion and pressure
Warnings
Do not use if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
- •
- heart disease
- •
- high blood pressure
- •
- thyroid disease
- •
- diabetes
- •
- trouble urinating due to an enlarged prostate gland
- •
- persistent or chronic cough such as occurs with smoking, asthma, chronic bronchitis, or emphysema
- •
- cough accompanied by too much phlegm (mucus)
Directions
- •
- do not crush, chew, or break extended-release tablet
- •
- take with a full glass of water
- •
- this product can be administered without regard for timing of meals
- •
- adults and children 12 years and older: 2 extended-release tablets every 12 hours; not more than 4 extended-release tablets in 24 hours
- •
- children under 12 years of age: do not use
Other information
Inactive ingredients
Questions?
PRINCIPAL DISPLAY PANEL - 36 Tablet Blister Pack Carton
Rugby®
NDC 0536-1333-36
Compare to the active
ingredients in Mucinex® D†
Guaifenesin and
Pseudoephedrine HCl
Extended-Release Tablets
Guaifenesin 600 mg
Pseudoephedrine HCl 60 mg
600 mg/60 mg
Expectorant and Nasal Decongestant
12 Hour
- •
- Clears Nasal/Sinus Congestion
- •
- Thins and Loosens Mucus
- •
- Immediate and Extended Release
Actual Size
36 Extended-Release Tablets
INGREDIENTS AND APPEARANCE
| GUAIFENESIN D
guaifenesin and pseudoephedrine hydrochloride tablet, extended release |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - RUGBY LABORATORIES, INC. (079246066) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Ohm Laboratories Inc. | 184769029 | MANUFACTURE(0536-1333) | |