Search by Drug Name or NDC
NDC 49348-0379-54 sunmark 2 g/100g Details
sunmark 2 g/100g
sunmark is a VAGINAL CREAM in the HUMAN OTC DRUG category. It is labeled and distributed by Strategic Sourcing Services LLC. The primary component is CLOTRIMAZOLE.
MedlinePlus Drug Summary
Vaginal clotrimazole is used to treat vaginal yeast infections in adults and children 12 years of age and older.. Clotrimazole is in a class of antifungal medications called imidazoles. It works by stopping the growth of fungi that cause infection.
Related Packages: 49348-0379-54Last Updated: 11/30/2022
MedLinePlus Full Drug Details: Clotrimazole Vaginal
Product Information
| NDC | 49348-0379 |
|---|---|
| Product ID | 49348-379_93126403-13fc-45c3-9605-bbdde72a1a2f |
| Associated GPIs | 55104020003710 |
| GCN Sequence Number | 013245 |
| GCN Sequence Number Description | clotrimazole CREAM/APPL 2 % VAGINAL |
| HIC3 | Q4F |
| HIC3 Description | VAGINAL ANTIFUNGALS |
| GCN | 28361 |
| HICL Sequence Number | 004137 |
| HICL Sequence Number Description | CLOTRIMAZOLE |
| Brand/Generic | Generic |
| Proprietary Name | sunmark |
| Proprietary Name Suffix | Clotrimazole 3 |
| Non-Proprietary Name | Clotrimazole |
| Product Type | HUMAN OTC DRUG |
| Dosage Form | CREAM |
| Route | VAGINAL |
| Active Ingredient Strength | 2 |
| Active Ingredient Units | g/100g |
| Substance Name | CLOTRIMAZOLE |
| Labeler Name | Strategic Sourcing Services LLC |
| Pharmaceutical Class | Azole Antifungal [EPC], Azoles [CS] |
| DEA Schedule | n/a |
| Marketing Category | NDA |
| Application Number | NDA021143 |
| Listing Certified Through | 2024-12-31 |
Package
Package Images
NDC 49348-0379-54 (49348037954)
| NDC Package Code | 49348-379-54 |
|---|---|
| Billing NDC | 49348037954 |
| Package | 1 TUBE, WITH APPLICATOR in 1 CARTON (49348-379-54) / 21 g in 1 TUBE, WITH APPLICATOR |
| Marketing Start Date | 2013-02-12 |
| NDC Exclude Flag | N |
| Pricing Information | |
| Price Per Unit | 0.32278 |
| Pricing Unit | GM |
| Effective Date | 2024-02-21 |
| NDC Description | SM 3-DAY VAGINAL CREAM |
| Pharmacy Type Indicator | C/I |
| OTC | Y |
| Explanation Code | 1, 5, 6 |
| Classification for Rate Setting | G |
| As of Date | 2024-02-21 |
This pricing file, entitled the NADAC (National Average Drug Acquisition
Cost) files, provide
state Medicaid agencies with covered outpatient drug prices by averaging
survey invoice
prices from retail community pharmacies across the United States. These
pharmacies include
independent retail community pharmacies and chain pharmacies. The prices
are updated on a
weekly and monthly basis
Standard Product Labeling (SPL)/Prescribing Information SPL 0bc5d91a-c4ea-4fe9-80e0-6f76aa073e7b Details
SPL UNCLASSIFIED SECTION
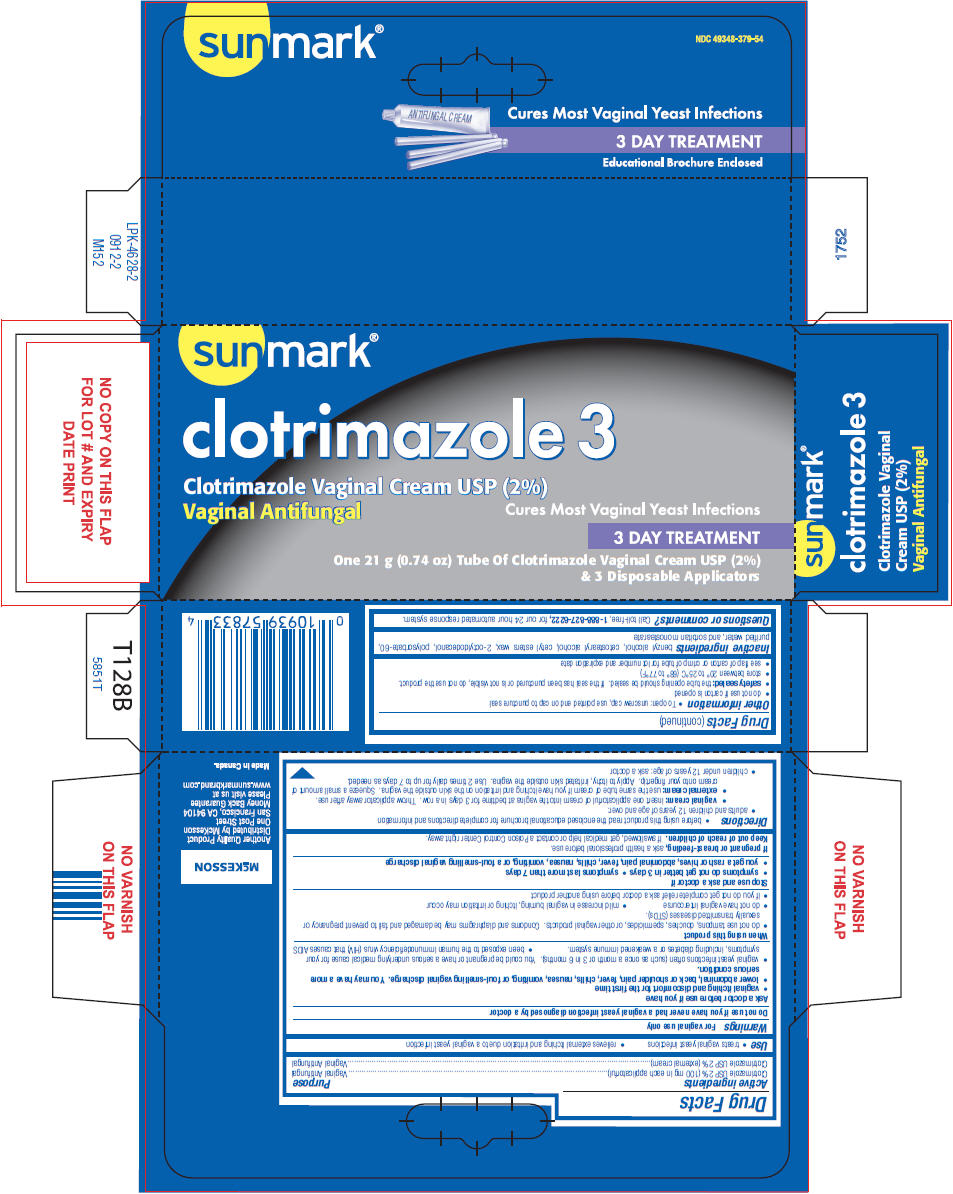
Use
Warnings
For vaginal use only
Ask a doctor before use if you have
- vaginal itching and discomfort for the first time
- lower abdominal, back or shoulder pain, fever, chills, nausea, vomiting, or foul-smelling vaginal discharge. You may have a more serious condition.
- vaginal yeast infections often (such as once a month or 3 in 6 months). You could be pregnant or have a serious underlying medical cause for your symptoms, including diabetes or a weakened immune system.
- been exposed to the human immunodeficiency virus (HIV) that causes AIDS
When using this product
- do not use tampons, douches, spermicides, or other vaginal products. Condoms and diaphragms may be damaged and fail to prevent pregnancy or sexually transmitted diseases (STDs).
- do not have vaginal intercourse
- mild increase in vaginal burning, itching or irritation may occur
- if you do not get complete relief ask a doctor before using another product
Directions
- before using this product read the enclosed educational brochure for complete directions and information
- adults and children 12 years of age and over:
- vaginal cream: insert one applicatorful of cream into the vagina at bedtime for 3 days in a row. Throw applicator away after use.
- external cream: use the same tube of cream if you have itching and irritation on the skin outside the vagina. Squeeze a small amount of cream onto your fingertip. Apply to itchy, irritated skin outside the vagina. Use 2 times daily for up to 7 days as needed.
- children under 12 years of age: ask a doctor
Other information
- To open: unscrew cap, use pointed end on cap to puncture seal
- do not use if carton is opened
- safety sealed: the tube opening should be sealed. If the seal has been punctured or is not visible, do not use the product.
- store between 20° to 25°C (68° to 77°F)
- see flap of carton or crimp of tube for lot number and expiration date
Inactive ingredients
PRINCIPAL DISPLAY PANEL - 21 g Tube Carton
INGREDIENTS AND APPEARANCE
| SUNMARK
CLOTRIMAZOLE 3
clotrimazole cream |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Strategic Sourcing Services LLC (116956644) |
| Registrant - Taro Pharmaceuticals U.S.A., Inc. (145186370) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Taro Pharmaceuticals Inc. | 206263295 | MANUFACTURE(49348-379) | |
Revised: 11/2019
Document Id: 93126403-13fc-45c3-9605-bbdde72a1a2f
Set id: 0bc5d91a-c4ea-4fe9-80e0-6f76aa073e7b
Version: 2
Effective Time: 20191121