Search by Drug Name or NDC
NDC 00143-9724-01 Clonidine Hydrochloride 100 ug/mL Details
Clonidine Hydrochloride 100 ug/mL
Clonidine Hydrochloride is a INTRAVENOUS INJECTION, SOLUTION in the HUMAN PRESCRIPTION DRUG category. It is labeled and distributed by Hikma Pharmaceuticals USA Inc.. The primary component is CLONIDINE HYDROCHLORIDE.
Product Information
| NDC | 00143-9724 |
|---|---|
| Product ID | 0143-9724_9d6244d1-5e85-4da3-b06c-f4fe8da1b6fa |
| Associated GPIs | 64200011102020 |
| GCN Sequence Number | 064360 |
| GCN Sequence Number Description | clonidine HCl/PF VIAL 1000MCG/10 EPIDURAL |
| HIC3 | H3C |
| HIC3 Description | ANALGESICS, NON-OPIOID |
| GCN | 13643 |
| HICL Sequence Number | 035805 |
| HICL Sequence Number Description | CLONIDINE HCL/PF |
| Brand/Generic | Generic |
| Proprietary Name | Clonidine Hydrochloride |
| Proprietary Name Suffix | n/a |
| Non-Proprietary Name | Clonidine Hydrochloride |
| Product Type | HUMAN PRESCRIPTION DRUG |
| Dosage Form | INJECTION, SOLUTION |
| Route | INTRAVENOUS |
| Active Ingredient Strength | 100 |
| Active Ingredient Units | ug/mL |
| Substance Name | CLONIDINE HYDROCHLORIDE |
| Labeler Name | Hikma Pharmaceuticals USA Inc. |
| Pharmaceutical Class | Adrenergic alpha2-Agonists [MoA], Central alpha-2 Adrenergic Agonist [EPC] |
| DEA Schedule | n/a |
| Marketing Category | ANDA |
| Application Number | ANDA200300 |
| Listing Certified Through | 2024-12-31 |
Package
Package Images




NDC 00143-9724-01 (00143972401)
| NDC Package Code | 0143-9724-01 |
|---|---|
| Billing NDC | 00143972401 |
| Package | 10 mL in 1 VIAL, SINGLE-USE (0143-9724-01) |
| Marketing Start Date | 2011-01-01 |
| NDC Exclude Flag | N |
| Pricing Information | N/A |
Standard Product Labeling (SPL)/Prescribing Information SPL 2a5b46ec-4c16-48a5-9e58-8d28a3f21274 Details
SPL UNCLASSIFIED SECTION
Rx Only
| The 500 mcg/mL strength product should be diluted prior to use in an appropriate solution. |
| NOTE: Clonidine hydrochloride injection (epidural clonidine) is not recommended for obstetrical, postpartum, or peri-operative pain management. The risk of hemodynamic instability, especially hypotension and bradycardia, from epidural clonidine may be unacceptable in these patients. However, in a rare obstetrical, post-partum or peri-operative patient, potential benefits may outweigh the possible risks. |
DESCRIPTION
Clonidine Hydrochloride Injection, USP is a centrally-acting analgesic solution for use in continuous epidural infusion devices.
Clonidine Hydrochloride, USP, is an imidazoline derivative and exists as a mesomeric compound. The chemical names are Benzenamine, 2, 6-dichloro-N-2-imidazolidinylidene monohydrochloride and 2-[(2,6-dichlorophenyl) imino]imidazolidine monohydrochloride. The following is the structural formula:

Clonidine Hydrochloride Injection, USP is supplied as a clear, colorless, preservative-free, pyrogen-free, aqueous sterile solution (pH 5 to 7) in 10 mL single-dose vials.
Each mL of the 1000 mcg/10 mL (0.1 mg/mL) concentration contains 100 mcg of Clonidine Hydrochloride, USP and 9 mg Sodium Chloride, USP in Water for Injection, USP. Hydrochloric acid and/or sodium hydroxide may have been added for pH adjustment. Each 10 mL vial contains 1 mg (1000 mcg) of clonidine hydrochloride.
Each mL of the 5000 mcg/10 mL (0.5 mg/mL) concentration contains 500 mcg of Clonidine Hydrochloride, USP and 9 mg Sodium Chloride, USP in Water for Injection, USP. Hydrochloric acid and/or sodium hydroxide may have been added for pH adjustment. Each 10 mL vial contains 5 mg (5000 mcg) of clonidine hydrochloride.
CLINICAL PHARMACOLOGY
Mechanism of Action
Epidurally administered clonidine produces dose-dependent analgesia not antagonized by opiate antagonists. The analgesia is limited to the body regions innervated by the spinal segments where analgesic concentrations of clonidine are present. Clonidine is thought to produce analgesia at presynaptic and postjunctional alpha-2-adrenoceptors in the spinal cord by preventing pain signal transmission to the brain.
Pharmacokinetics
Following a 10 minute intravenous infusion of 300 mcg clonidine HCl to five male volunteers, plasma clonidine levels showed an initial rapid distribution phase (mean ± SD t1/2= 11 ± 9 minutes) followed by a slower elimination phase (t1/2= 9 ± 2 hours) over 24 hours. Clonidine’s total body clearance (CL) was 219 ± 92 mL/min.
Following a 700 mcg clonidine HCl epidural dose given over five minutes to four male and five female volunteers, peak clonidine plasma levels (4.4 ± 1.4 ng/mL) were obtained in 19 ± 27 minutes. The plasma elimination half-life was determined to be 22 ± 15 hours following sample collection for 24 hours. CL was 190 ± 70 mL/min. In cerebral spinal fluid (CSF), peak clonidine levels (418 ± 255 ng/mL) were achieved in 26 ± 11 minutes. The clonidine CSF elimination half-life was 1.3 ± 0.5 hours when samples were collected for 6 hours. Compared to men, women had a lower mean plasma clearance, longer mean plasma half-life, and higher mean peak level of clonidine in both plasma and CSF.
In cancer patients who received 14 days of clonidine HCl epidural infusion (rate = 30 mcg/hr) plus morphine by patient-controlled analgesia (PCA), steady state clonidine plasma concentrations of 2.2 ± 1.1 and 2.4 ± 1.4 ng/mL were obtained on dosing days 7 and 14, respectively. CL was 279 ± 184 and 272 ± 163 mL/min on these days. CSF concentrations were not determined in these patients.
Distribution
Clonidine is highly lipid soluble and readily distributes into extravascular sites including the central nervous system. Clonidine’s volume of distribution is 2.1 ± 0.4 L/kg. The binding of clonidine to plasma protein is primarily to albumin and varies between 20 and 40% in vitro. Epidurally administered clonidine readily partitions into plasma via the epidural veins and attains systemic concentrations (0.5 – 2.0 ng/mL) that are associated with a hypotensive effect mediated by the central nervous system.
Excretion
Following an intravenous dose of 14C-clonidine, 72% of the administered dose was excreted in urine in 96 hours of which 40 - 50% was unchanged clonidine. Renal clearance for clonidine was determined to be 133 ± 66 mL/min. In a study where 14C-clonidine was given to subjects with varying degrees of kidney function, elimination half-lives varied (17.5 to 41 hours) as a function of creatinine clearance. In subjects undergoing hemodialysis only 5% of body clonidine stores were removed.
Metabolism
In humans, clonidine metabolism follows minor pathways with the major metabolite, p-hydroxy-clonidine, being present at less than 10% of the concentration of unchanged drug in urine.
Special Populations
The pharmacokinetics of epidurally administered clonidine has not been studied in the pediatric population or in patients with renal or hepatic disease.
Clinical Trials
In a double-blind, randomized study of cancer patients with severe intractable pain below the C4 dermatome not controlled by morphine, 38 patients were randomized to an epidural infusion of clonidine hydrochloride injection plus epidural morphine, whereas 47 subjects received epidural placebo plus epidural morphine. Both groups were allowed rescue doses of epidural morphine. Successful analgesia, defined as a decrease in either morphine use or Visual Analog Score (VAS) pain, was significantly more common with epidural clonidine than placebo (45% vs 21%, p=0.016). Only the subgroup of 36 patients with “neuropathic” pain, characterized by the investigator as well-localized, burning, shooting, or electric-like pain in a dermatomal or peripheral nerve distribution had significant analgesic effects relative to placebo in this study.
The most frequent adverse events with clonidine were hypotension (45% vs 11% for placebo, p<0.001), postural hypotension (32% vs 0%, p<0.001), dizziness (13% vs 4%, p=0.234), anxiety (11% vs 2%, p=0.168) and dry mouth (13% vs 9%, p=0.505). Both mean blood pressure and heart rate were reduced in the clonidine group. At the conclusion of the two-week study period in the clinical trial, all patients were abruptly withdrawn from study drug or placebo. Four patients of the clonidine group suffered rebound hypertension upon withdrawal of clonidine; one of these patients suffered a cerebrovascular accident. Asymptomatic bradycardia was noted in one clonidine patient.
INDICATIONS AND USAGE
Clonidine Hydrochloride Injection, USP is indicated in combination with opiates for the treatment of severe pain in cancer patients that is not adequately relieved by opioid analgesics alone. Epidural clonidine is more likely to be effective in patients with neuropathic pain than somatic or visceral pain (see CLINICAL PHARMACOLOGY: Clinical Trials).
The safety of this drug product has only been established in a highly selected group of cancer patients, and only after an adequate trial of opioid analgesia. Other use is of unproven safety and is not recommended. In a rare patient, the potential benefits may outweigh the known risks (see WARNINGS).
CONTRAINDICATIONS
Clonidine hydrochloride injection is contraindicated in patients with a history of sensitization or allergic reactions to clonidine. Epidural administration is contraindicated in the presence of an injection site infection, in patients on anticoagulant therapy, and in those with a bleeding diathesis. Administration of clonidine hydrochloride injection above the C4 dermatome is contraindicated since there are no adequate safety data to support such use (see WARNINGS).
WARNINGS
Use in Postoperative or Obstetrical Analgesia
Clonidine hydrochloride injection (epidural clonidine) is not recommended for obstetrical, post-partum, or peri-operative pain management. The risk of hemodynamic instability, especially hypotension and bradycardia, from epidural clonidine may be unacceptable in these patients.
Hypotension
Because severe hypotension may follow the administration of clonidine, it should be used with caution in all patients. It is not recommended in most patients with severe cardiovascular disease or in those who are otherwise hemodynamically unstable. The benefit of its administration in these patients should be carefully balanced against the potential risks resulting from hypotension.
Vital signs should be monitored frequently, especially during the first few days of epidural clonidine therapy. When clonidine is infused into the upper thoracic spinal segments, more pronounced decreases in the blood pressure may be seen.
Clonidine decreases sympathetic outflow from the central nervous system resulting in decreases in peripheral resistance, renal vascular resistance, heart rate, and blood pressure. However, in the absence of profound hypotension, renal blood flow and glomerular filtration rate remain essentially unchanged.
In the pivotal double-blind, randomized study of cancer patients, where 38 subjects were administered epidural clonidine hydrochloride injection at 30 mcg/hr in addition to epidural morphine, hypotension occurred in 45% of subjects. Most episodes of hypotension occurred within the first four days after beginning epidural clonidine. However, hypotensive episodes occurred throughout the duration of the trial. There was a tendency for these episodes to occur more commonly in women, and in those with higher serum clonidine levels. Patients experiencing hypotension also tended to weigh less than those who did not experience hypotension. The hypotension usually responded to intravenous fluids and, if necessary, appropriate parenterally-administered pressor agents.
Published reports on the use of epidural clonidine for intraoperative or postoperative analgesia also show a consistent and marked hypotensive response to clonidine. Severe hypotension may occur even if intravenous fluid pretreatment is given.
Withdrawal
Sudden cessation of clonidine treatment, regardless of the route of administration, has, in some cases, resulted in symptoms such as nervousness, agitation, headache, and tremor, accompanied or followed by a rapid rise in blood pressure. The likelihood of such reactions appears to be greater after administration of higher doses or with concomitant beta-blocker treatment. Special caution is therefore advised in these situations. Rare instances of hypertensive encephalopathy, cerebrovascular accidents and death have been reported after abrupt clonidine withdrawal. Patients with a history of hypertension and/or other underlying cardiovascular conditions may be at particular risk of the consequences of abrupt discontinuation of clonidine. In the pivotal double-blind, randomized cancer pain study, four of 38 subjects receiving 720 mcg of clonidine per day experienced rebound hypertension following abrupt withdrawal. One of these patients with rebound hypertension subsequently experienced a cerebrovascular accident.
Careful monitoring of infusion pump function and inspection of catheter tubing for obstruction or dislodgement can help reduce the risk of inadvertent abrupt withdrawal of epidural clonidine. Patients should notify their physician immediately if clonidine administration is inadvertently interrupted for any reason. Patients should also be instructed not to discontinue therapy without consulting their physician.
When discontinuing therapy with epidural clonidine, the physician should reduce the dose gradually over 2 to 4 days to avoid withdrawal symptoms.
An excessive rise in blood pressure following discontinuation of epidural clonidine can be treated by administration of clonidine or by intravenous phentolamine. If therapy is to be discontinued in patients receiving a beta-blocker and clonidine concurrently, the beta-blocker should be withdrawn several days before the gradual discontinuation of epidural clonidine.
PRECAUTIONS
General
Cardiac Effects: Epidural clonidine frequently causes decreases in heart rate. Symptomatic bradycardia can be treated with atropine. Rarely, atrioventricular block greater than first degree has been reported. Clonidine does not alter the hemodynamic response to exercise, but may mask the increase in heart rate associated with hypovolemia.
Respiratory Depression and Sedation: Clonidine administration may result in sedation through the activation of alpha-adrenoceptors in the brainstem. High doses of clonidine cause sedation and ventilatory abnormalities that are usually mild. Tolerance to these effects can develop with chronic administration. These effects have been reported with bolus doses that are significantly larger than the infusion rate recommended for treating cancer pain.
Depression: Depression has been seen in a small percentage of patients treated with oral or transdermal clonidine. Depression commonly occurs in cancer patients and may be exacerbated by treatment with clonidine. Patients, especially those with a known history of affective disorders, should be monitored for the signs and symptoms of depression.
Pain of Visceral or Somatic Origin: In the clinical investigations, at doses tested, clonidine hydrochloride injection was most effective in well-localized, “neuropathic” pain that was characterized as electrical, burning, or shooting in nature, and which was localized to a dermatomal or peripheral nerve distribution. Clonidine hydrochloride injection may be less effective, or possibly ineffective in the treatment of pain that is diffuse, poorly localized, or visceral in origin.
Information for Patients
Patients should be instructed about the risks of rebound hypertension and warned not to discontinue clonidine except under the supervision of a physician. Patients should notify their physician immediately if clonidine administration is inadvertently interrupted for any reason. Patients who engage in potentially hazardous activities, such as operating machinery or driving, should be advised of the potential sedative and hypotensive effects of epidural clonidine. They should also be informed that sedative effects may be increased by CNS-depressing drugs such as alcohol and barbiturates, and that hypotensive effects may be increased by opiates.
Drug Interactions
Clonidine may potentiate the CNS-depressive effect of alcohol, barbiturates or other sedating drugs. Narcotic analgesics may potentiate the hypotensive effects of clonidine. Tricyclic antidepressants may antagonize the hypotensive effects of clonidine. The effects of tricyclic antidepressants on clonidine’s analgesic actions are not known.
Beta-blockers may exacerbate the hypertensive response seen with clonidine withdrawal. Also, due to the potential for additive effects such as bradycardia and AV block, caution is warranted in patients receiving clonidine with agents known to affect sinus node function or AV nodal conduction, e.g., digitalis, calcium channel blockers, and beta-blockers.
There is one reported case of a patient with acute delirium associated with the simultaneous use of fluphenazine and oral clonidine. Symptoms resolved when clonidine was withdrawn and recurred when the patient was rechallenged with clonidine.
Epidural clonidine may prolong the duration of pharmacologic effects of epidural local anesthetics, including both sensory and motor blockade.
Carcinogenesis, Mutagenesis, Impairment of Fertility
In a 132-week study in rats, clonidine hydrochloride administered as a dietary admixture at 5-8 times (based on body surface area) the 50 mcg/kg maximum recommended daily human dose (MRDHD) for hypertension did not show any carcinogenic potential. Clonidine was inactive in the Ames test of mutagenicity. Fertility of male or female rats was unaffected by oral clonidine hydrochloride doses as high as 150 mcg/kg, or about 0.5 times the MRDHD. Fertility of female rats did, however, appear to be affected in another experiment at oral dose levels of 500-2000 mcg/kg, or 2-7 times the MRDHD.
Usage in Pregnancy/Teratogenic Effects
Reproduction studies in rabbits at clonidine hydrochloride doses up to approximately the MRDHD revealed no evidence of teratogenic or embryotoxic potential. In rats, however, doses as low as one-third the MRDHD were associated with increased resorptions in a study in which dams were treated continuously from 2 months prior to mating. Increased resorptions were not associated with treatment with the same or higher doses up to 0.5 times the MRDHD when dams were treated on days 6-15 of gestation. Increased resorptions were observed at higher levels (7-times the MRDHD) in rats and mice treated on days 1-14 of gestation.
Clonidine readily crosses the placenta and its concentrations are equal in maternal and umbilical cord plasma; amniotic fluid concentrations can be 4 times those found in serum. There are no adequate and well-controlled studies in pregnant women during early gestation when organ formation takes place. Studies using epidural clonidine during labor have demonstrated no apparent adverse effects on the infant at the time of delivery. However, these studies did not monitor the infants for hemodynamic effects in the days following delivery. Clonidine hydrochloride injection should be used during pregnancy only if the potential benefits justify the potential risk to the fetus.
Labor and Delivery
There are no adequate controlled clinical trials evaluating the safety, efficacy, and dosing of clonidine hydrochloride injection in obstetrical settings. Because maternal perfusion of the placenta is critically dependent on blood pressure, use of clonidine hydrochloride as an analgesic during labor and delivery is not indicated (see WARNINGS).
Nursing Mothers
Concentrations of clonidine in human breast milk are approximately twice those found in maternal plasma. Caution should be exercised when clonidine is administered to a nursing woman. Because of the potential for severe adverse reactions in nursing infants, a decision should be made to either discontinue nursing or to discontinue clonidine.
Pediatric Use
The safety and effectiveness of clonidine hydrochloride injection in this limited indication and clinical population have been established in patients old enough to tolerate placement and management of an epidural catheter, based on evidence from adequate and well controlled studies in adults and experience with the use of clonidine in the pediatric age group for other indications. The use of clonidine hydrochloride injection should be restricted to pediatric patients with severe intractable pain from malignancy that is unresponsive to epidural or spinal opiates or other more conventional analgesic techniques. The starting dose of clonidine hydrochloride injection should be selected on per kilogram basis (0.5 mcg per kg per hour) and cautiously adjusted based on the clinical response.
ADVERSE REACTIONS
Adverse reactions seen during continuous epidural clonidine infusion are dose-dependent and typical for a compound of this pharmacologic class. The adverse events most frequently reported in the pivotal controlled clinical trial of continuous epidural clonidine administration consisted of hypotension, postural hypotension, decreased heart rate, rebound hypertension, dry mouth, nausea, confusion, dizziness, somnolence, and fever. Hypotension is the adverse event that most frequently requires treatment. The hypotension is usually responsive to intravenous fluids and, if necessary, appropriate parenterally-administered pressor agents. Hypotension was observed more frequently in women and in lower weight patients, but no dose-related response was established.
Implantable epidural catheters are associated with a risk of catheter-related infections, including meningitis and/or epidural abscess. The risk depends on the clinical situation and the type of catheter used, but catheter related infections occur in 5%-20% of patients, depending on the kind of catheter used, catheter placement technique, quality of the catheter care, and length of catheter placement.
The inadvertent intrathecal administration of clonidine has not been associated with a significantly increased risk of adverse events, but there are inadequate safety and efficacy data to support the use of intrathecal clonidine.
Epidural clonidine was compared to placebo in a two week double-blind study of 85 terminal cancer patients with intractable pain receiving epidural morphine. The following adverse events were reported in two or more patients and may be related to administration of either clonidine hydrochloride injection or morphine.
| Adverse Events | Clonidine
N = 38 n (%) | Placebo
N = 47 n (%) |
| Total Number of Patients Who Experienced at Least One Adverse Event | 37 (97.4) | 38 (80.5) |
| Hypotension | 17 (44.8) | 5 (10.6) |
| Postural Hypotension | 12 (31.6) | 0 (0) |
| Dry Mouth | 5 (13.2) | 4 ( 8.5) |
| Nausea | 5 (13.2) | 10 (21.3) |
| Somnolence | 5 (13.2) | 10 (21.3) |
| Dizziness | 5 (13.2) | 2 (4.3) |
| Confusion | 5 (13.2) | 5 (10.6) |
| Vomiting | 4 (10.5) | 7 (14.9) |
| Nausea/Vomiting | 3 (7.9) | 1 (2.1) |
| Sweating | 2 (5.3) | 0 (0) |
| Chest Pain | 2 (5.3) | 0 (0) |
| Hallucination | 2 (5.3) | 1 (2.1) |
| Tinnitus | 2 (5.3) | 0 (0) |
| Constipation | 1 (2.6) | 2 (4.3) |
| Tachycardia | 1 (2.6) | 2 (4.3) |
| Hypoventilation | 1 (2.6) | 2 (4.3) |
An open label long-term extension of the above trial was performed. Thirty-two subjects received epidural clonidine and morphine for up to 94 weeks with a median dosing period of 10 weeks. The following adverse events (and percent incidence) were reported: hypotension/postural hypotension (47%); nausea (13%); anxiety/confusion (38%); somnolence (25%); urinary tract infection (22%); constipation, dyspnea, fever, infection (6% each); asthenia, hyperaesthesia, pain, skin ulcer, and vomiting (5% each). Eighteen percent of subjects discontinued this study as a result of catheter-related problems (infections, accidental dislodging, etc.), and one subject developed meningitis, possibly as a result of catheter-related infection. In this study, rebound hypertension was not assessed, and ECG and laboratory data not systematically sought.
The following adverse reactions have also been reported with the use of any dosage form of clonidine. In many cases patients were receiving concomitant medication and a causal relationship has not been established:
Body as a Whole: Weakness, 10%; fatigue, 4%; headache and withdrawal syndrome, each 1%. Also reported were pallor, a weakly positive Coomb’s test, and increased sensitivity to alcohol.
Cardiovascular: Palpitations and tachycardia, and bradycardia, each 0.5%. Syncope, Raynaud’s phenomenon, congestive heart failure, and electrocardiographic abnormalities (i.e., sinus node arrest, functional bradycardia, high degree AV block) have been reported rarely. Rare cases of sinus bradycardia and atrioventricular block have been reported, both with and without the use of concomitant digitalis.
Central Nervous System: Nervousness and agitation, 3%; mental depression, 1%; insomnia, 0.5%. Cerebrovascular accidents, other behavioral changes, vivid dreams or nightmares, restlessness, and delirium have been reported rarely.
Dermatological: Rash, 1%; pruritus, 0.7%; hives, angioneurotic edema and urticaria, 0.5%; alopecia, 0.2%.
Gastrointestinal: Anorexia and malaise, each 1%; mild transient abnormalities in liver function tests, 1%; hepatitis, parotitis, ileus and pseudo obstruction, and abdominal pain, rarely.
Genitourinary: Decreased sexual activity, impotence, and libido, 3%; nocturia, about 1%; difficulty in micturition, about 0.2%; urinary retention, about 0.1%.
Hematologic: Thrombocytopenia, rarely.
Metabolic: Weight gain, 0.1%; gynecomastia, 1%; transient elevation of glucose or serum phosphatase, rarely.
Musculoskeletal: Muscle or joint pain, about 0.6%; leg cramps, 0.3%.
Oro-otolaryngeal: Dryness of the nasal mucosa was rarely reported.
Ophthalmological: Dryness of the eyes, burning of the eyes and blurred vision were rarely reported.
To report SUSPECTED ADVERSE REACTIONS, contact Hikma Pharmaceuticals USA Inc. at 1-877-845-0689 or the FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
OVERDOSAGE
Hypertension may develop early and may be followed by hypotension, bradycardia, respiratory depression, hypothermia, drowsiness, decreased or absent reflexes, irritability, and miosis. With large oral overdoses, reversible cardiac conduction defects or arrhythmias, apnea, coma, and seizures have been reported. As little as 100 mcg of oral clonidine has produced signs of toxicity in pediatric patients.
There is no specific antidote for clonidine overdosage. Supportive care may include atropine sulfate for bradycardia, intravenous fluids and/or vasopressor agents for hypotension. Hypertension associated with overdosage has been treated with intravenous furosemide, diazoxide or alpha-blocking agents such as phentolamine. Naloxone may be a useful adjunct in the treatment of clonidine-induced respiratory depression, hypotension, and/or coma; blood pressure should be monitored since the administration of naloxone has occasionally resulted in paradoxical hypertension. Tolazoline administration has yielded inconsistent results and is not recommended as first-line therapy. Dialysis is not likely to significantly enhance the elimination of clonidine.
The largest overdose reported to date involved a 28-year old white male who ingested 100 mg of clonidine hydrochloride powder. This patient developed hypertension, followed by hypotension, bradycardia, apnea, hallucinations, semi-coma, and premature ventricular contractions. The patient fully recovered after intensive treatment. Plasma clonidine levels were 60 ng/mL after 1 hour, 190 ng/mL after 1.5 hours, 370 ng/mL after 2 hours, and 120 ng/mL after 5.5 and 6.5 hours. In mice and rats, the oral LD50 of clonidine is 206 and 465 mg/kg, respectively.
DOSAGE AND ADMINISTRATION
The recommended starting dose of clonidine hydrochloride injection for continuous epidural infusion is 30 mcg/hr. Although dosage may be titrated up or down depending on pain relief and occurrence of adverse events, experience with dosage rates above 40 mcg/hr is limited.
Familiarization with the continuous epidural infusion device is essential. Patients receiving epidural clonidine from a continuous infusion device should be closely monitored for the first few days to assess their response.
The 500 mcg/mL (0.5 mg/mL) strength product must be diluted prior to use in 0.9% Sodium Chloride for Injection, USP, to a final concentration of 100 mcg/mL:
| Volume of Clonidine Hydrochloride Injection
500 mcg/mL | Volume of 0.9% Sodium Chloride for Injection, USP | Resulting Final Clonidine Hydrochloride Injection Concentration
(100 mcg/mL) |
| 1 mL | 4 mL | 500 mcg/5 mL |
| 2 mL | 8 mL | 1000 mcg/10 mL |
| 3 mL | 12 mL | 1500 mcg/15 mL |
| 4 mL | 16 mL | 2000 mcg/20 mL |
| 5 mL | 20 mL | 2500 mcg/25 mL |
| 6 mL | 24 mL | 3000 mcg/ 30 mL |
| 7 mL | 28 mL | 3500 mcg/ 35 mL |
| 8 mL | 32 mL | 4000 mcg/40 mL |
| 9 mL | 36 mL | 4500 mcg/45 mL |
| 10 mL | 40 mL | 5000 mcg/50 mL |
Renal Impairment: Dosage should be adjusted according to the degree of renal impairment, and patients should be carefully monitored. Since only a minimal amount of clonidine is removed during routine hemodialysis, there is no need to give supplemental clonidine following dialysis.
Clonidine hydrochloride injection must not be used with a preservative.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
HOW SUPPLIED
NDC 0143-9724-01, 100 mcg/mL solution in 10 mL vials, packaged individually.
NDC 0143-9723-01, 500 mcg/mL solution in 10 mL vials, packaged individually.
Store at 25ºC (77ºF); excursions permitted to 15º to 30ºC (59º to 86ºF) [See USP Controlled Room Temperature].
Preservative Free. Discard unused portion.
Manufactured by:
HIKMA FARMACÊUTICA (PORTUGAL), S.A.
Estrada do Rio da Mó, 8, 8A e 8B – Fervença
2705 – 906 Terrugem SNT
PORTUGAL
Distributed by:
Hikma Pharmaceuticals USA Inc.
Berkeley Heights, NJ 07922
Revised: May 2022
PIN265-WES/2
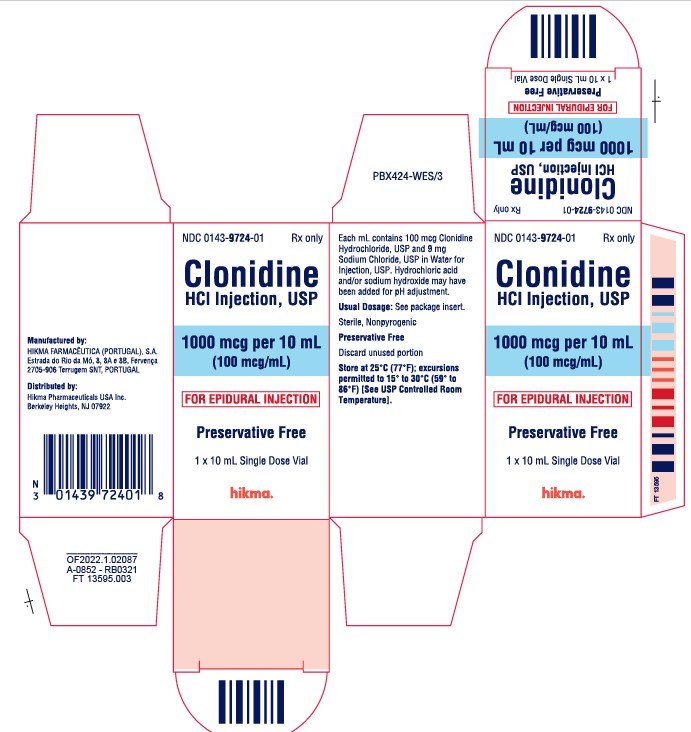
PRINCIPAL DISPLAY PANEL
NDC 0143-9724-01 Rx only
Clonidine HCl Injection, USP
1000 mcg per 10 mL
(100 mcg/mL)
FOR EPIDURAL INJECTION
Preservative Free
10 mL Single Dose Vial

NDC 0143-9724-01 Rx only
Clonidine HCl Injection, USP
1000 mcg per 10 mL
(100 mcg/mL)
FOR EPIDURAL INJECTION
Preservative Free
1 x 10 mL Single Dose Vial

PRINCIPAL DISPLAY PANEL
NDC 0143-9723-01 Rx only
Clonidine HCl Injection, USP
5000 mcg per 10 mL
(500 mcg/mL)
FOR EPIDURAL INJECTION
MUST BE DILUTED BEFORE USE
Preservative Free
10 mL Single Dose Vial

NDC 0143-9723-01 Rx only
Clonidine HCl Injection, USP
5000 mcg per 10 mL
(100 mcg/mL)
FOR EPIDURAL INJECTION
MUST BE DILUTED BEFORE USE
Preservative Free
1 x 10 mL Single Dose Vial

INGREDIENTS AND APPEARANCE
| CLONIDINE HYDROCHLORIDE
clonidine hydrochloride injection, solution |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| CLONIDINE HYDROCHLORIDE
clonidine hydrochloride injection, solution |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - Hikma Pharmaceuticals USA Inc. (001230762) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| HIKMA FARMACEUTICA (PORTUGAL), S.A | 452742943 | manufacture(0143-9724, 0143-9723) | |